The properties and toxicity assessment of homosalate
Background
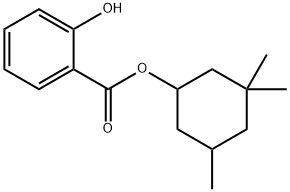
Homosalate (3,3,5‐trimethylcyclohexylsalicylate; Figure 1) is an organic UV filter that absorbs UVB radiation at wavelengths from 290 to 320 nm, that can be found in many cosmetics. The presence of alkyl‐substituted cyclohexane ring in UV filters can considerably alter the lipophilicity of the whole molecule which might possibly have a significant effect on metabolism. Studies have shown that homosalate passes by systemic circulation after topical application of a gel formulation in rats. Hence, HMS has been shown to cause hormonaldys function when absorbed in in vivo and in vitro studies. HMS accumulates in aquatic biota and humans through the food chain;therefore, it is important to consider the effects on health and know its mechanisms of action. Although the daily use of UV filters are low, long-term exposure to UV filters can cause cytotoxic and genotoxic effects.[1] The maximum allowable concentration of homosalate in final products varies in different countries but is relatively high, i.e., it is15% in the USA and Australia and 10% in EU, Korea, and Japan. With awareness of the harmful effects of exposure to UV radiation, the use of sunscreens and UV filtering preparations has been increased over the years.In a cohort study conducted in Switzerland, 77.36% of lactating mothers reported the use of sunscreens and other cosmetics containing UV filters.[2]In particular, 15.09% of the lactating mothers used homosalate-containing sunscreens and cosmetics. It may be reasonable to speculate that the use of sunscreens and UV filtering cosmetics is also prevalent in the general population. Homosalate has been recently reported to accumulate in the aquatic systems of the surface water of streams and rivers and their polluted sediments.[2]

Chemical and physical properties
Homosalate (CAS no. 118-56-9) is a clear, colourless to pale yellow liquid with a molecular weight of 262.3 g/mol and a partition coefficient of 6.34 (at 40°C). The substance is miscible in paraffin oil, isopropyl myristate and ethanol (at 20°C), and immiscible in propylene glycol (at 20°C). Solubility in water (at 25°C) is 0.4 mg/L.
Toxicity Assessment
Toxicokinetics (Taken from Scientific Committee on Consumer Safety (SCCS)/1622/20 )
Several in vitro dermal penetration studies using rat and human skin have been performed.For MoS calculation, the SCCS selected a new skin penetration study using human skin from which a dermal absorption of 5.3% (mean + 1SD: 3.86±1.43) was derived. Systemic bioavailability of homosalate after dermal application was confirmed by the detection of homosalate in plasma of volunteers after topical application of sunscreen products containing homosalate but also by the detection of homosalate human milk samples. Maximum plasma concentrations of homosalate after topical application varied between 13.9 and 23.1 ng/ml and terminal half-lives varied between 46.9 and 78.4 h in an explorative study. In vitro, homosalate was hydrolysed into salicylic acid and 3,3,5-trimethylcyclohexanol. In addition,conjugation and hydroxylation of intact homosalate was observed.[3]
Four volunteers applied a commercial sunscreen containing 10% homosalate to their whole body under regular-use conditions (18-40 mg HMS (kg bw)−1). Parent homosalate isomers and hydroxylated and carboxylic acid metabolites were quantified using authentic standards and isotope dilution analysis. Further metabolites were investigated semi-quantitatively. Elimination was delayed and slower compared to the oral route, and terminal elimination half-times were around 24 h. After dermal exposure, the bioavailability of cis-homosalate was a factor of 2 lower than that of trans-homosalate. However, metabolite ratios in relation to the respective parent isomer were very similar to the oral route, supporting the applicability of the oral-route urinary excretion fractions for dermal-route exposure assessments. Exemplary calculations of intake doses showed margins of safety between 11 and 92 (depending on the approach) after single whole-body sunscreen application. Human biomonitoring can reliably quantify oral and dermal homosalate exposures and support the monitoring of exposure reduction measures.[4]
Assessment of the cytotoxicity and genotoxicity of homosalate in MCF‐7
Methods: Cell viability was examined by the 3‐(4,5‐dimethylthiazol‐2‐yl)2,5‐diphenyl tetrazolium bromide (MTT) and cell membrane integrity by the lactate dehydroge‐ nase release assays (LDH), and genotoxicity by using the micronucleus test at 250, 500, 750, 1000, 1500, and 2000 µM concentrations with the human breast cell line MCF-7.
Results: Homosalate affected the cell viability dose‐dependently at a concentrations of above 1000 µM. Micronucleus formation was significantly induced at 750 and 1000 µM within 24 hours due to an increase in cytostatic effect, the cell viability of HMS decreased to 57% at a concentration of 2000 µM, and a sufficient number of binucleated cells could not be obtained to count. Homosalate was also clastogenic when the cells were incubated at cytotoxic concentrations. These results suggest that homosalate can be considered as a cytotoxic and genotoxic substance.[1]
Homosalate aggravates the invasion of human trophoblast cells
Yang et al. evaluated if HTR8/SVneo, a human trophoblast cell line, treated with homosalate showed decreasing proliferative activity in a dose-dependent manner. Homosalate promoted the death of HTR8/SVneo cells with elevated lipid peroxidation and intracellular Ca2+ concentration. It also induced endoplasmic reticulum stress and mitochondrial morphological disturbances associated with the differentiation of human trophoblast cells. However, when the intracellular Ca2+ or reactive oxygen species were removed using BAPTA-AM or N-acetyl L-cysteine (NAC), the cell proliferation suppressed by homosalate was restored. Homosalate also significantly inhibited the invasion of HTR8/SVneo cells. Furthermore, it modulated phosphoinositide 3-kinase (PI3K)/AKT and mitogen-activated protein kinase (MAPK) signaling pathways, which were involved in the cross-talk between both signaling pathways in HTR8/SVneo cells. Thus, homosalate adversely affects the survival, proliferation, and invasiveness of human trophoblast cells and therefore pregnant women should practice caution while using personal care products containing homosalate.[5]
Conclusion
Although the daily usage of UV filters is low, the long-term exposure to organic UV filters can cause to serious toxic effects including cytotoxicity and genotoxicity. Keeping this in mind, it would be important and necessary to carry out extensive studies on toxicological effects. UV filters should be examined thoroughly for risk assessment. Additionally, the toxic effects of UV filters should be extensively evaluated by the regulatory authorities and cosmetics companies.
References
[1] Yazar S, Kara Ertekin S. Assessment of the cytotoxicity and genotoxicity of homosalate in MCF-7. J Cosmet Dermatol. 2020;19(1):246-252. doi:10.1111/jocd.12973
[2] Kim T H , Shin B S , Shin S W ,et al.HPLC-MS/MS method for the pharmacokinetics of homosalate after topical administration in rats[J].Journal of Liquid Chromatography & Related Technologies, 2014, 37(17):2465-2477.DOI:10.1080/10826076.2013.840842.
[3] Scientific Committee on Consumer Safety. Scientific advice on the safety of Homosalate (CAS No 118-56-9,EC No 204-260-8) as a UV-filter in cosmetic products[EB/OL].(2021-12-02)[2022-05-11].https://ec.europa.eu/health/document/download/40b8ecf8-7c93-4a7b-b257-1fc16533ba31_en?filename=sccs_o_260.pdf
[4] Ebert KE, Griem P, Weiss T, et al. Toxicokinetics of homosalate in humans after dermal application: applicability of oral-route data for exposure assessment by human biomonitoring. Arch Toxicol. 2024;98(5):1383-1398. doi:10.1007/s00204-024-03704-7
[5] Yang C, Lim W, Bazer FW, Song G. Homosalate aggravates the invasion of human trophoblast cells as well as regulates intracellular signaling pathways including PI3K/AKT and MAPK pathways. Environ Pollut. 2018;243(Pt B):1263-1273. doi:10.1016/j.envpol.2018.09.092
You may like
See also
Lastest Price from Homosalate manufacturers

US $0.00-0.00/KG2025-07-05
- CAS:
- 118-56-9
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KGS

US $0.00-0.00/kg2025-06-11
- CAS:
- 118-56-9
- Min. Order:
- 0.001kg
- Purity:
- 99%
- Supply Ability:
- 2000000T