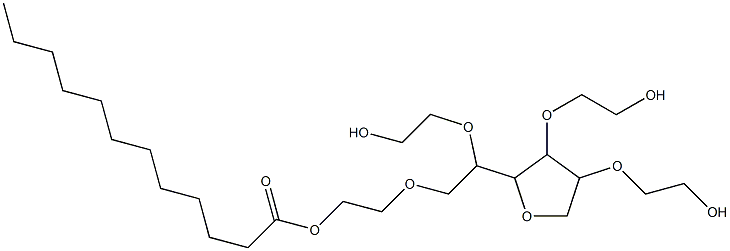
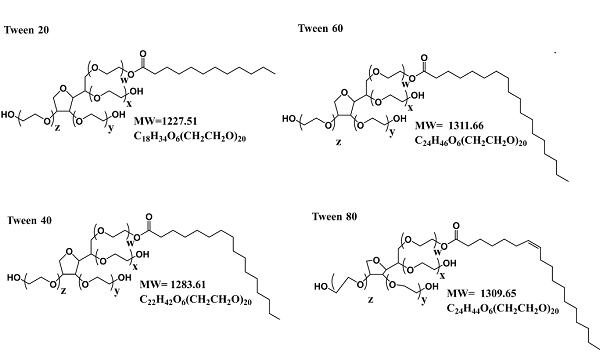
The preparation method of polysorbate 20
Background
Polysorbate 20 is a commonly used excipient in biopharmaceutical formulations, some of which may have an enzymatic activity[1]. The action(s) of polysorbate 20 in biopharmaceutical formulations as a stabilizer require this surfactant to maintain its intact structure. This manuscript evaluates a new analytic method for the analysis of polysorbate 20 degradation in the format of a biopharmaceutical formulation and makes a comparison with several established methods of analysis.
Picture 1 One bottle of polysorbate 20
The preparation method of polysorbate 20
Polysorbate 20 samples were degraded in a controlled environment utilizing the enzyme pancreatic lipase to generate degradants that included lauric acid and the sorbitan polyoxyethylene side chain. A new method was developed with sufficient sensitivity to analyze the degraded solutions. Lauric acid was derivatized with the fluorescent reagent 9-anthryldiazomethane to form 9-anthrylmethylethyl ester. The derivatized lauric acid was separated by reversed-phase chromatography and detected by fluorescence or UV spectroscopy. Three established methods utilized to measure polysorbate 20 were evaluated for their ability to detect degraded polysorbate 20. These methods were: (1) fluorescence analysis with N-phenyl-1-naphthylamine fluorescent dye; (2) UV spectroscopy with ammonium cobaltothiocyanate colorimetric reagent; and (3) nuclear magnetic resonance (NMR).
Polysorbate 20 incubation with lipase resulted in degraded polysorbate 20 as determined by the derivatized lauric acid assay. The UV spectroscopy assay utilizing ammonium cobaltothiocyanate reagent was not able to detect the degradation of polysorbate 20 in the samples. The fluorescence method of analysis detected polysorbate 20 degradation as an approximate 50% decrease in micelles in comparison to standard nondegraded polysorbate 20 solutions. NMR analysis resulted in similar proton peak areas for both degraded and nondegraded polysorbate 20 samples. NMR spectra did contain minor differences between the samples.
It is essential to choose the appropriate method of polysorbate 20 evaluation to assess the content, stability, and compatibility of a formulation. Current established methods to assess polysorbate 20 may overlook and do not necessarily monitor the potential degradation of this surfactant, which results in the formation of lauric acid. Because this type of degradation may occur in a formulation by an enzymatically active biopharmaceutical, a new method of analysis has been established.
The CMC of polysorbate 20
The CMC of polysorbate 20 was determined using a surface tension method[2]; the concentration (C) of polysorbate 20 studied varied from 0.001 to 10.000 mg./ml. The results show clearly that the surface tension (γ) decreases linearly with log C up to a concentration of 0.06 mg./ml. and is practically constant for more concentrated solutions. This suggests that the CMC of polysorbate 20 is in the vicinity of 0.06 mg./ml., which is in excellent agreement with the values obtained by other methods.
Application
Monoclonal antibodies (MAbs) are widely used as therapeutic proteins and they are frequently exposed to a high degree of stress during manufacturing or delivery[3]. MAbs shear thin upon increasing shear rates. After undergoing multiple shear cycles, with a cone-and-plate rheometer, the solution viscosity of high concentration antibodies increases due to the formation of insoluble aggregates. These shear-induced insoluble aggregates do not form when polysorbate 20 is present in solution. We hypothesize that monoclonal antibodies form a thin protein layer at the air-water interface. MAbs at the interface expose their hydrophobic core to air leading to unfolding, multiple non-specific intermolecular interactions and, upon continuous high shear, precipitation. Surface tension analysis confirms that monoclonal antibodies are surface active and that polysorbate 20 can prevent their interaction with the air-water interface. In addition, we complement these findings with a viscometer that measures bulk viscosity without the influence of an air-liquid interfacial viscosity and find that the bulk viscosity increases slightly when Mab solutions contained polysorbate 20. These methods of analysis could be used when designing manufacturing systems in which a protein solution is subject to shear forces.
Reference
1 Khossravi M, Kao Y H, Mrsny R J, et al. Analysis methods of polysorbate 20: A new method to assess the stability of polysorbate 20 and established methods that may overlook degraded polysorbate 20[J]. Pharmaceutical research, 2002, 19(5): 634-639.
2 Mittal K L. Determination of CMC of polysorbate 20 in aqueous solution by surface tension method[J]. Journal of pharmaceutical sciences, 1972, 61(8): 1334-1335.
3 Patapoff T W, Esue O. Polysorbate 20 prevents the precipitation of a monoclonal antibody during shear[J]. Pharmaceutical development and technology, 2009, 14(6): 659-664.
Related articles And Qustion
See also
Lastest Price from Polysorbate 20 manufacturers

US $150.00/kg2025-11-25
- CAS:
- 9005-64-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $0.00-0.00/kg2025-05-30
- CAS:
- 9005-64-5
- Min. Order:
- 1kg
- Purity:
- 98%min
- Supply Ability:
- 20mt