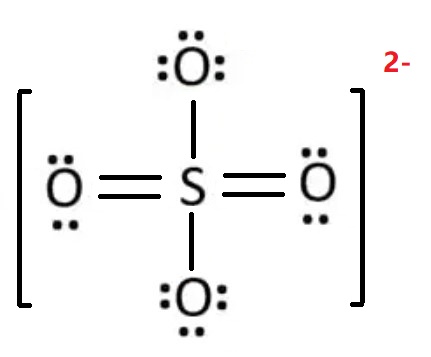
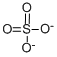
The Lewis structure of Sulfate Standard
Description
The sulfate ion (Sulfate Standard) is a polyatomic anion with the empirical formula SO42-. Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid, and many are prepared from that acid.
The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state, while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2, and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, HSO4-, which is, in turn, the conjugate base of H2SO4, sulfuric acid.
Lewis structure
Step 1: Total valence electrons in SO42- ion
First, determine the valence electron available for drawing the Lewis structure of SO42-because the Lewis diagram represents valence electrons around atoms. Sulfur and oxygen atoms are located in the VIA group in the periodic table. So, oxygen and sulfur atoms have six electrons in their valence shell.
Total valence electrons valence electrons given by one sulfur atom + valence electrons given by four oxygen atoms+2 more electrons are added due to 2 negative charges = 6 + 6*(4)+2 = 32.
Step 2: Total valence electrons pairs
Total valance electron pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number of total valence electrons by two. For SO42- ion, the Total pairs of electrons are 16.
Step 3: Select the central atom
In theory, the atom which is less electronegative remains at the center. Compare the electronegativity values of sulfur (S) and oxygen (O); the sulfur atom is less electronegative. Hence, the sulfur atom (S) is the center atom, and the oxygen atoms (O) are the outside atoms.
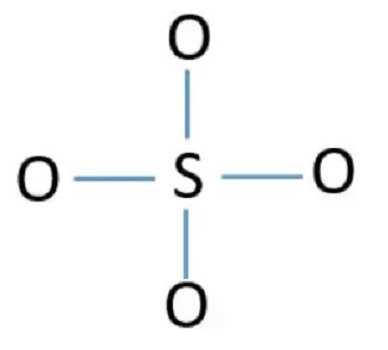
Step 4: Connect outer atoms to the central atom with a single bond and place electron pairs between the atoms
There are already four S-O keys in the sketch above. This leaves only 12 valence electron pairs that must be placed around the atom. The remaining electron pairs are first placed around the outer oxygen atoms. Thus, the following figure can be obtained.
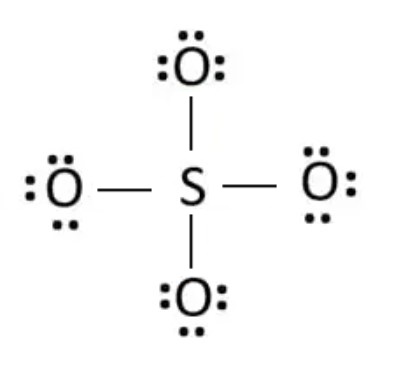
Step 5: Charges on atoms
After marking electron pairs on atoms, we should mark the charges of each atom. Each oxygen atom will get a -1 charge, and the sulfur atom get a +2 charge. Hence, the overall charge of the ion is ( -1*4 + (+2) ) = -2.
Step 6: Check the stability of the Lewis structure
The stability of Lewis's structure can be checked using the concept of a formal charge.
Formal charge = Valence electrons – (Bonding electrons)/2 – Nonbonding electrons
For Sulfur (S) atom:
Formal charge=6-8/2-0=+2
For oxygen atom:
Formal charge=6-2/2-6=-1
When charges exist everywhere (on atoms) in the ion or molecule, that structure is not stable. We should minimize charges by converting lone pairs or pairs to bonds. So, convert one lone pair of electrons of one oxygen atom to make a new S-O bond. The charges on sulfur and two oxygen atoms become zero after shifting the electron pair from the oxygen atom to the sulfur atom. This is a more stable Lewis structure (see image below). In addition, there is a -1 formal charge on the other two oxygen atoms. -1 + -1 = -2, which accounts for an overall negative 2 charge on the sulfate SO42- ion. This ensures a correct and stable Lewis representation for the sulfate SO42- ion. S atom can accommodate more than 8 valence electrons during chemical bond formation due to the presence of 3d orbitals in its atomic structure.
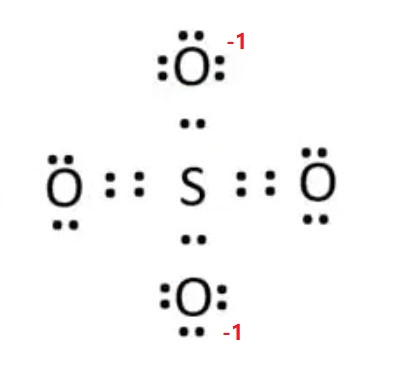
Hence, the Lewis structure of SO42-is shown below: