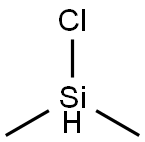
Chlorodimethylsilane: applications in organic synthesis and safety
General Description
Chlorotriethoxysilane is a versatile compound with crucial roles in various applications. It serves as a key component in the synthesis of spherosilicate dendrimer DMS-1, enabling the creation of well-defined silica-based nanoparticles with exceptional potential in nanotechnology. Additionally, chlorotriethoxysilane plays a significant role in deoxygenative chlorination reactions, catalyzing the production of chlorination products with superior selectivity and efficiency. Furthermore, it facilitates the chlorination of alcohols under mild conditions, showcasing its versatility in synthetic chemistry. However, it is important to handle chlorotriethoxysilane with caution due to its corrosive nature and potential hazards, emphasizing the need for proper safety measures and handling procedures in industrial applications.

Figure 1. Chlorodimethylsilane
Applications
Synthesis of spherosilicate dendrimer DMS-1
Chlorotriethoxysilane, a key component in the synthesis of spherosilicate dendrimer DMS-1, plays a crucial role in various applications due to its unique properties. DMS-1, featuring closely spaced reaction sites and 24 reactive Si-H groups on its surface, is synthesized through stepwise silylation with chlorotriethoxysilane and chlorodimethylsilane. With a diameter of approximately 1.5 nm, DMS-1 represents the smallest well-defined silica-based nanoparticle, offering exceptional potential in nanotechnology. Furthermore, DMS-1 exhibits versatility, forming molecular crystals and demonstrating solubility in common organic solvents. Its surface can be modified via hydrosilylation with different reagents, thereby expanding its applicability. Notably, DMS-1 serves as a foundational element for nanohybrids and stands out as the most precisely designed siloxane-based nanoparticle available. Additionally, by heating a cast film of DMS-1 at 180 °C for 5 days, a molecularly ordered silica-based hybrid can be obtained, further broadening its scope of applications. In conclusion, chlorotriethoxysilane's contribution to the synthesis and properties of DMS-1 underscores its significance in the development of advanced nanomaterials with diverse functional capabilities. 1
Deoxygenative chlorination reactions
Chlorotriethoxysilane plays a crucial role in various chemical reactions and applications. One significant application is its use as a reagent in deoxygenative chlorination reactions. Chlorotriethoxysilane is involved in the catalytic reaction of carbonyls and chlorodimethylsilane. The reaction, effectively catalyzed by indium(III) hydroxide, results in the production of deoxygenative chlorination products. In this process, the carbonyl carbon accepts two nucleophiles (hydrogen and chlorine) while releasing oxygen. Remarkably, only In(OH)3 catalyzes the reaction, demonstrating superior catalytic activity compared to typical Lewis acids such as TiCl4, AlCl3, and BF3.OEt2. The mechanism of this deoxygenative chlorination involves initial hydrosilylation followed by chlorination. Notably, other nucleophiles such as allyl or iodine can also be utilized in this methodology. The moderate Lewis acidity of the indium catalyst enables chemoselective reactions, ensuring that ester, nitro, cyano, or halogen groups remain unaffected during the reaction course. Overall, chlorotriethoxysilane's role in these catalytic reactions highlights its significance in enabling selective and efficient deoxygenative chlorination processes with broad applicability in organic synthesis. 2
Chlorination
Chlorotriethoxysilane plays a crucial role in various chemical reactions. One notable application is its use in the InCl3-catalyzed chlorination of alcohols in the presence of benzil. This process results in the formation of organic chlorides under mild conditions. Benzil's presence significantly influences the reaction course, leading to the production of the desired chlorinated products. Furthermore, chlorotriethoxysilane enables the effective chlorination of secondary or tertiary alcohols, making it a versatile reagent in synthetic chemistry. Additionally, the system utilizing chlorotriethoxysilane allows for the selective chlorination of tertiary sites, even in the presence of primary alcohols, showcasing its high degree of selectivity. The highly coordinated hydrosilane formed from benzil and HSiMe2Cl serves as a critical intermediate in this process. Overall, chlorotriethoxysilane demonstrates its significance as a facilitator of selective and efficient chlorination reactions, making it an essential component in organic synthesis. 3
Safety
Chlorotriethoxysilane is a chemical compound commonly used in industrial applications, such as in the production of adhesives, coatings, and sealants. It is important to handle chlorotriethoxysilane with caution due to its potential hazards. This compound is corrosive and can cause severe skin and eye irritation upon contact. Inhalation of its vapors can lead to respiratory irritation and damage. Therefore, it is crucial to use proper personal protective equipment, such as gloves, goggles, and a respirator, when working with chlorotriethoxysilane. Additionally, it should be stored in a well-ventilated area away from incompatible substances to prevent accidents or reactions. Regular safety training and thorough understanding of handling procedures are essential to minimize risks associated with chlorotriethoxysilane. 4
Reference
1. Kawahara K, Hagiwara Y, Kuroda K. Dendritic, nanosized building block for siloxane-based materials: a spherosilicate dendrimer. Chemistry. 2011;17(47):13188-13196.
2. Onishi Y, Ogawa D, Yasuda M, Baba A. Direct conversion of carbonyl compounds into organic halides: indium(III) hydroxide-catalyzed deoxygenative halogenation using chlorodimethylsilane. J Am Chem Soc. 2002;124(46):13690-13691.
3. Yasuda M, Yamasaki S, Onishi Y, Baba A. Indium-catalyzed direct chlorination of alcohols using chlorodimethylsilane-benzil as a selective and mild system. J Am Chem Soc. 2004;126(23):7186-7187.
4. Chlorodimethylsilane. European Chemicals Agency, EC / List no. 213-912-0.
You may like
Related articles And Qustion
See also
Lastest Price from Chlorodimethylsilane manufacturers
US $0.00-0.00/kg2025-12-09
- CAS:
- 1066-35-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $79.00-38.00/kg2025-04-21
- CAS:
- 1066-35-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton