2-Bromofluorene: properties, applications and safety
General Description
2-Bromofluorene is a halogenated aromatic compound with a molecular formula C13H9Br, belonging to the class of fluorenes. It is a white to off-white solid with a melting point of 67-69°C and a boiling point of 323-325°C, insoluble in water but soluble in organic solvents. Chemically, it exhibits typical reactivity of aromatic compounds and is used in organic synthesis to create OLED materials and pharmaceutical intermediates. However, it is classified as a probable human carcinogen and should be handled with caution. Proper storage, personal protective equipment, and waste disposal procedures are necessary to minimize risks and maintain a safe working environment.

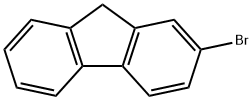
Figure 1. 2-Bromofluorene
Properties
2-Bromofluorene is a chemical compound with a molecular formula C13H9Br. It belongs to the class of organic compounds known as fluorenes, which are polycyclic aromatic hydrocarbons. This compound is characterized by the presence of a bromine atom attached to the fluorine ring of the fluorene structure. In terms of physical properties, 2-Bromofluorene is a solid with a white to off-white color. It has a melting point of around 67-69°C and a boiling point of approximately 323-325°C. It is insoluble in water but soluble in common organic solvents such as acetone and ethanol. Chemically, 2-Bromofluorene exhibits reactivity typical of aromatic compounds. It can undergo various reactions, including substitution, addition, and oxidation reactions. The bromine atom attached to the fluorene ring makes it susceptible to nucleophilic substitution reactions. This compound finds application in various fields, including organic synthesis and materials science. It can be used as a building block for the synthesis of more complex organic compounds. Additionally, its bromine substituent can impart specific properties to polymers and materials. Overall, 2-Bromofluorene is a chemically versatile compound with distinct physical and chemical properties, making it valuable in diverse applications within the field of organic chemistry and materials science. 1
Applications
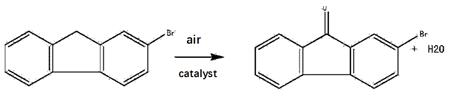
2-Bromofluorene is a versatile chemical compound with applications in organic synthesis, materials science, and pharmaceuticals. In organic synthesis, it is used to synthesize novel ter(9,9-diarylfluorene)s through Suzuki-coupling reactions, resulting in compounds with enhanced thermal and morphological stability, intense blue fluorescence, and interesting reversible redox properties. These compounds have potential applications in OLED devices as emitters and hole transporters. In materials science, 2-Bromofluorene serves as a precursor for the synthesis of fluorescent polymers and OLED materials. It exhibits excellent thermal stability and electron transport properties, making it suitable for use in optoelectronic devices. In the pharmaceutical industry, 2-Bromofluorene is utilized in the synthesis of biologically active compounds, including pharmaceutical intermediates and drug candidates. Its unique structure and reactivity offer opportunities for the development of novel drugs with improved therapeutic properties. Overall, 2-Bromofluorene is a valuable compound that finds applications across different scientific disciplines. Its versatility and usefulness make it an important tool for researchers in organic synthesis, materials science, and pharmaceutical development. 2
Safety
2-Bromofluorene is a halogenated aromatic compound with potential safety concerns. It is classified as a probable human carcinogen by the EPA based on animal studies. The compound should be handled with caution and used under controlled conditions. Long-term exposure to high concentrations of 2-Bromofluorene can lead to adverse health effects, including irritation and cancer. To ensure safety, it is important to follow exposure limits and use personal protective equipment when working with this compound. In terms of storage, 2-Bromofluorene is flammable and should be stored in a cool, well-ventilated area away from heat and open flames. Direct contact or inhalation of the compound can cause irritation to the skin, eyes, and respiratory system. Therefore, it is necessary to wear protective gear such as gloves, goggles, and a lab coat when handling it. Swallowing the compound should be avoided, and proper hand hygiene must be practiced after handling it. In case of accidental exposure, immediate medical attention is required. Proper waste disposal procedures should also be followed to prevent environmental contamination. Overall, strict adherence to safety protocols, including storage, handling, and disposal, is crucial to minimize the risks associated with 2-Bromofluorene and maintain a safe working environment. 3
Reference
1. PubChem. COMPOUND SUMMARY: 2-Bromofluorene. National Library of Medicine, PubChem CID: 14336.
2. Wong KT, Chien YY, Chen RT, Wang CF, Lin YT, Chiang HH, Hsieh PY, Wu CC, Chou CH, Su YO, Lee GH, Peng SM. Ter(9,9-diarylfluorene)s: highly efficient blue emitter with promising electrochemical and thermal stability. J Am Chem Soc. 2002 Oct 2;124(39):11576-11577.
3. Zirconium tetrabutanolate. European Chemicals Agency, EC / List no. 213-995-3.
Related articles And Qustion
See also
Lastest Price from 2-Bromofluorene manufacturers

US $210.00/KG2025-04-21
- CAS:
- 1133-80-8
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 300MT/year
US $14.00-123.00/g2025-02-08
- CAS:
- 1133-80-8
- Min. Order:
- 100g
- Purity:
- 0.98
- Supply Ability:
- 10kg