The introduction of Tamoxifen
Description
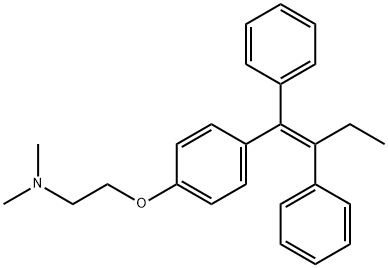
Tamoxifen (TAM), also known as trans-1-(4-β-dimethylamino ethoxy phenyl)-1,2-diphenylbut-1-ene, is a small organic compound with the chemical formula C26H29NO. This compound is soluble in methanol, ethanol, propanol, or propylene glycol, but insoluble in water. Tamoxifen is considered a groundbreaking drug in medical oncology that has saved many lives over the past four decades and progressed to become a significant part of our healthcare.
History
In the late 1950s, in the laboratories of Imperial Chemical Industries Ltd. Pharmaceutical division now known as AstraZeneca, a team with Dora Richardson (chemist), Michael J. K. Harper (reproductive endocrinologist), and Arthur L. Walpole (Head of Reproduction Research) was given the task of developing a post-coital contraceptive (the morning-after pill). Eventually, TAM (Imperial Chemical Industries (ICI) 46 474) was invented and received marketing approval as a fertility treatment. However, this drug never actually proved useful in human contraception and played an important role in cancer treatment. In 1972, Walpole collaborated with V.C. Jordan on scientific research that reinvented the failed ICI 46474 contraceptive pill as tamoxifen, the first targeted drug for the treatment and prevention of breast cancer.
Biological function
As a selective estrogen receptor modulator (SERM), this substance could compete with estradiol in binding to estrogen receptors and inhibit the binding of the receptor to the estrogen-response element on DNA. TAM also plays a role in the upregulation of transforming growth factor B (TGF-B) and downregulation of insulin-like growth factor 1 (IGF-1), hence playing a significant role in the suppression of tumor cell growth, particularly in breast cancer. Pharmacological studies of tamoxifen in the human body suggested its conversion to three active metabolites: 4-hydroxy tamoxifen, N-desmethyltamoxifen (primary metabolite), and 4-hydroxy-N-desmethyltamoxifen, also known as endoxifen.
Uses
TAM is commonly used for treating pre- and perimenopausal women with estrogen receptor-positive breast cancer and is also commonly used as a chemopreventive in women at moderate to high risk of breast cancer. An analysis from the National Health Interview Survey (NHIS) showed that approximately 0.03% of women without breast cancer in the United States used TAM as a chemopreventive agent. Anovulatory failure and gynecomastia are other indications for TAM.
Side effects
As there are estrogen receptors in the retina, retinal pigment epithelium, and choroid, these tissues may also be affected by TAM. Hence, this drug may cause ocular adverse effects (such as ocular surface disorders, cataracts, retinopathy, and optic neuropathy). Retinopathy is the most known and well-studied ocular adverse effect of TAM therapy.
References
Related articles And Qustion
See also
Lastest Price from Tamoxifen manufacturers

US $10.00/box2025-07-31
- CAS:
- 10540-29-1
- Min. Order:
- 1box
- Purity:
- 99.99%
- Supply Ability:
- 9999

US $5.00-0.50/KG2025-05-07
- CAS:
- 10540-29-1
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS