Dimethyl 1,3-acetonedicarboxylate: properties and applications in organic synthesis
General Description
Dimethyl 1,3-acetonedicarboxylate is a highly reactive compound with excellent solubility. It has been extensively used in organic synthesis for various applications. When combined with different reactants, it undergoes reactions that lead to the formation of functionalized compounds with potential applications in sunscreen agents for UV protection. Additionally, it plays a role in chemo- and stereoselective reactions, allowing for the synthesis of highly functionalized molecules that can be used as synthetic intermediates in the development of pharmaceuticals and agrochemicals. Furthermore, it is employed in the synthesis of functionally embellished o-Alkynylbenzoates or Furan-3(2H)-ones, providing versatile building blocks for the creation of complex molecules with diverse functionality. Overall, Dimethyl 1,3-acetonedicarboxylate showcases its synthetic potential and expands the range of accessible chemical space in organic synthesis.

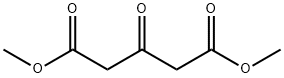
Figure 1. Dimethyl 1,3-acetonedicarboxylate
Properties
Dimethyl 1,3-acetonedicarboxylate is a chemical compound with interesting properties. Firstly, it has a molecular formula of C7H10O5 and a molecular weight of 174.15 g/mol. Dimethyl 1,3-acetonedicarboxylate is a colorless liquid with a fruity odor. One notable property is its high reactivity. It undergoes various chemical reactions, such as esterification, transesterification, and condensation reactions. Furthermore, Dimethyl 1,3-acetonedicarboxylate exhibits good solubility in polar organic solvents like methanol and ethanol. It is also stable under normal conditions, with a boiling point of 236-238 °C and a flash point of 125 °C. In conclusion, Dimethyl 1,3-acetonedicarboxylate is a highly reactive compound with excellent solubility. 1
Applications in organic synthesis
Combination with various reactants
Dimethyl 1,3-acetonedicarboxylate, in combination with various reactants such as 3-methoxalyl, 3-polyfluoroacyl, and 3-aroylchromones, as well as 1,3-diphenylacetone, can undergo reactions in the presence of DBU (1,8-diazabicyclo[5.4.0]undec-7-ene). These reactions primarily occur at the C-2 atom of the chromone system. The reactions result in pyrone ring-opening and subsequent formal [3 + 3] cyclocondensation, leading to the formation of functionalized 2-hydroxybenzophenones, 6H-benzo[c]chromenes, and benzo[c]coumarins. The specific compound synthesized depends on the substituent present at the 3-position of the reactant. The application of these reactions extends to the design and development of novel UV-A/B and UV-B filters. The synthesized compounds can be considered as potential scaffolds for creating new sunscreen agents that protect against harmful ultraviolet (UV) radiation. To further understand the structures and properties of the synthesized compounds, a study involving Nuclear Magnetic Resonance (NMR) analysis and X-ray crystallographic analysis has been conducted, providing valuable insights into their characteristics. Overall, this research demonstrates the utility of Dimethyl 1,3-acetonedicarboxylate in synthesizing functional compounds with potential applications in the field of sunscreens and UV protection. 2
Chemo- and stereoselective reaction
Dimethyl 1,3-acetonedicarboxylate finds application in the chemo- and stereoselective reaction between alkyl isocyanides and itself in the presence of acetylenic esters. When alkyl isocyanides react with dimethyl 1,3-acetonedicarboxylate in the presence of dialkyl acetylenedicarboxylates, under ambient temperature conditions and in CH(2)Cl(2) solvent, a transformation occurs. This reaction results in the formation of highly functionalized 2-amino-4H-pyrans and 1,2-dialkyl 4,6-dimethyl-(1E, 3E)-3 (alkylamino)-5-oxo-1,3-hexadiene-1,2,4,6-tetracarboxylates. The process exhibits both chemo- and stereoselectivity, meaning that specific chemical bonds are selectively formed, and the desired stereochemistry is achieved. The resulting compounds possess intricate structures containing amino and carbonyl groups, making them valuable synthetic intermediates.By utilizing dimethyl 1,3-acetonedicarboxylate in this reaction, chemists can access these unique and functionalized molecules, expanding the toolbox for organic synthesis. The ability to selectively modify and functionalize these compounds holds great potential for the development of novel pharmaceuticals, agrochemicals, and other bioactive molecules. 3
Synthesis of o-akynylbenzoates or fran-3(2H)-ones
Dimethyl 1,3-acetonedicarboxylate is employed in the synthesis of functionally embellished o-Alkynylbenzoates or Furan-3(2H)-ones from diynones. This innovative approach allows for controlled product diversification through a one-pot reaction between diynones and dimethyl 1,3-acetonedicarboxylate. The reaction conditions dictate whether the desired outcome is the formation of functionally unique pentasubstituted o-alkynylbenzoates or fully substituted furan-3(2H)-ones. The selectivity of the reaction enables chemists to tailor the synthesis towards their specific needs. These two resulting platforms offer versatility and the ability to enter new utilitarian chemical space. The functionally embellished o-Alkynylbenzoates and fully substituted furan-3(2H)-ones are valuable building blocks in organic synthesis. They can serve as precursors for the creation of complex molecules with diverse functionality and potential pharmacological activities. By utilizing the reactivity of dimethyl 1,3-acetonedicarboxylate in this reaction, researchers can access these functionalized compounds, providing new opportunities for the development of drugs, agrochemicals, and other fine chemicals. This approach showcases the synthetic potential of dimethyl 1,3-acetonedicarboxylate in expanding the range of accessible chemical space. 4
Reference
1. PubChem. COMPOUND SUMMARY: Diethyl 1,3-acetonedicarboxylate. National Library of Medicine, 2005, CID: 66045.
2. Iaroshenko VO, Savych I, Villinger A, Sosnovskikh VY, Langer P. Reactions of 3-acylchromones with dimethyl 1,3-acetonedicarboxylate and 1,3-diphenylacetone: one-pot synthesis of functionalized 2-hydroxybenzophenones, 6H-benzo[c]chromenes and benzo[c]coumarins. Org Biomol Chem. 2012 Dec 21;10(47):9344-9348.
3. Nasiri F, Nazem F, Pourdavaie K. Chemo- and stereoselective reaction between alkyl isocyanides and dimethyl 1,3-acetonedicarbocxylate in the presence of acetylenic esters. Mol Divers. 2007 May;11(2):101-105.
4. Sarma MJ, Sudarshana KA, Srihari P, Mehta G. An Approach to Functionally Embellished o-Alkynylbenzoates or Furan-3(2H)-ones from Diynones and DMAD: Controlled Divergence and Product Selectivity. J Org Chem. 2023 Mar 17;88(6):3945-3953.
Related articles And Qustion
See also
Lastest Price from Dimethyl 1,3-acetonedicarboxylate manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 1830-54-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT

US $30.00-10.00/KG2025-04-15
- CAS:
- 1830-54-2
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg