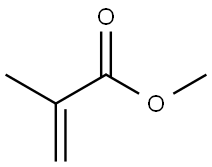
The introduction of Poly(methyl methacrylate)
Description
The polymethyl methacrylate (PMMA; Poly(methyl methacrylate)) macromolecule is based on a monomer that corresponds to an ethylene molecule with one hydrogen atom substituted by a methyl group (i.e., CH3 —), while the second hydrogen atom on the same carbon is replaced by an acetyl group (i.e., CH3 COO—) giving the basic monomer unit [—CH3 —C(CH3 )(COOCH3 )—]n.

Synthesis method
The raw chemical intermediate used for making polymethyl methacrylate is 2-hydroxy-2-methyl-propane nitrile, which is prepared by reacting acetone with hydrocyanic acid according to the following reaction:
CH3 COCH3 + HCN → (CH3 )2 C(OH)CN
Afterward, the 2-hydroxy-2-methylpropanenitrile is reacted with methanol (CH3 OH) to yield the methacrylate ester.
Another former route consisted of reacting methylacrylic acid [CH2 =C(CH3 )COOCH3 ] directly with methanol. The polymerization reaction is initiated by organic peroxides or azo catalysts to produce the polymethyl methacrylate macromolecule finally. The polymerization is highly exothermic, as indicated by the elevated enthalpy of the reaction (58 kJ.mol–1). Therefore, the process requires fast heat removal from the reactor vessel by efficient cooling. Continuous or batch processes are used. The batch process yields higher molecular weight PMMA, while the continuous process leads to copolymers.
Uses
The commercial product is well known under the common trade name Plexiglas®. PMMA is a clear and rigid thermoplastic; it is also readily formed by injection molding. The main applications are guards and covers. Generally, the good atmospheric stability and clarity of acrylics have made them useful as high-impact window panes and other see-through barriers significant in the industry. Various modifications are being made to alter the properties of the basic acrylic resins for specific services. However, these are not of great use in the industrial area. The upper temperature of usefulness is approximately 90°C. The loss in light transmission is only 1% after five years of exposure in locations with high sunlight exposure.
References
[1] Materials Handbook, DOI 10.1007/978-1-84628-669-8.
You may like
See also
Lastest Price from Poly(methyl methacrylate) manufacturers
US $0.00-0.00/KG2025-11-18
- CAS:
- 9011-14-7
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/g2025-04-21
- CAS:
- 9011-14-7
- Min. Order:
- 5g
- Purity:
- ≥98.0%
- Supply Ability:
- 10kg/month