The effects of fluralaner on dogs
Background
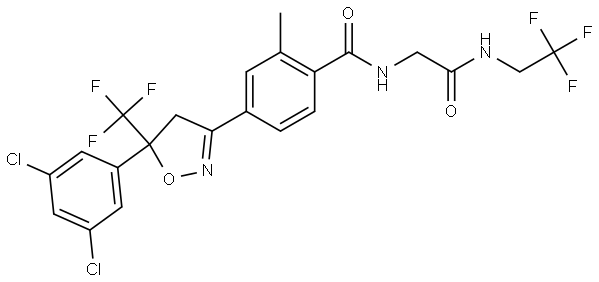
Fluralaner is a novel systemically administered insecticidal and acaricidal compound that provides long-acting efficacy after oral administration to dogs. Fluralaner belongs to a new class of compounds, the isoxazolines. A field study has shown that a single fluralaner dose administered orally to dogs provides at least twelve weeks of flea- and tick-control [1]. The long duration of activity offers a more convenient treatment over monthly flea and tick control treatments with a potential compliance advantage, reducing the risk of vector-transmitted diseases.
Feeding affects gastrointestinal physiology and therefore may influence the pharmacokinetics of a concurrently administered drug through reduced, delayed, increased, or accelerated absorption; in addition, there are drugs with no food interaction [2–4]. Altered pharmacokinetics may have an impact on the clinical activity of fluralaner [3, 4]. For example, an increase in absorption in a fed animal may lead to a prolonged period of efficacy. Administration of fluralaner tablets at or around the time of feeding may be an option chosen by some dog owners to facilitate administration of the chewable tablet. A drug-food effect cannot be predicted on a scientific basis and specific studies are required to investigate possible effects [2–4]; therefore, this study was performed to evaluate the impact of food on the pharmacokinetic profile of fluralaner chewable tablets.
Method
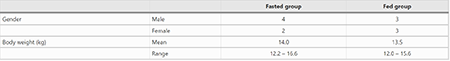
This study was conducted in compliance with the German animal protection legislation framework and ethical approval was obtained before the start of the study (authorization reference 23 177-07 /G 08-4-003). Dogs were kept indoors in pens with sealed floors and individually housed until 3 days after tablet administration. They had access to water ad libitum throughout the study period and were fed a standard dog diet (Bosch Tiernahrung GmbH&Co. KG; dry kibble food; composition: protein 21%, fat 6%, crude fiber 7%, crude ash 6%, moisture 10%). 12 healthy beagles were allocated to either a fed or fasted study group by sorting dogs within gender according to ascending body weight and alternately assigning dogs to a group (Table 1).
table 1 Fasted and fed dog groups for evaluation of fluralaner pharmacokinetic parameters
Result
All dogs were dosed once orally with 25 mg fluralaner/kg body weight on day 0 using chewable tablets containing fluralaner. Chewable tablets were cut to achieve the individual target dose. Tablets were placed on the back of the tongue and swallowing was stimulated with a small amount of tap water.
Both groups were fasted for 25 hours prior to fluralaner administration. Dogs in the fed group were offered half the normal daily food ration 15 minutes prior to fluralaner administration and the remaining half of the daily food ration was offered to these dogs immediately after administration. Dogs in the fasted group received orally administered fluralaner chewable tablets without feeding and remained unfed for a further 8 hours. Dogs were observed at regular intervals for any clinical findings throughout the study. The veterinary study supervisor assessed any clinical findings for their relationship to fluralaner treatment. All treatment-related findings were classified as adverse events.
Blood samples for fluralaner plasma concentration determination were collected prior to tablet administration and at 2, 4, and 8 hours, and then 1, 2, 3, 4, 7, 14, 21, 28, 42, 56, 70, 84, and 91 days after tablet administration. The blood sampling time points were selected based on previous pharmacokinetic data (unpublished observations), to cover initial rapid absorption, redistribution and prolonged elimination over 13 weeks following oral treatment. Fluralaner blood plasma concentrations were determined using automated solid-phase extraction coupled to liquid chromatography with mass-spectrometry (Online-SPE – HPLC/MS/MS; lower limit of quantification 5 ng/ml). The bioanalytical method was validated based on regulatory guidelines [5, 6].
Fluralaner pharmacokinetics evaluation was based on the plasma concentration of the parent compound for the area under the curve from time 0 to the last sampling time point associated with a quantifiable concentration (91 days after administration, AUC0-91d), maximum plasma concentration (Cmax) and time to Cmax (tmax). Pharmacokinetic parameters were calculated using noncompartmental methods (WinNonlin 5.2.1, Pharsight, Mountain View, California). The AUC0-91d was calculated using the linear trapezoidal method.
The effect of food on fluralaner pharmacokinetics was calculated by comparison of the means of AUC0-91d and Cmax of both groups according to the following ratios: AUC0-91d(fed)/AUC0-91d(fasted) or Cmax(fed)/Cmax(fasted). Statistics (SAS® Language: Reference, Version 9.3, SAS Institute Inc., Cary, NC, USA) were calculated using the individual dog as the experimental unit.
Reference
1 Rohdich N, Roepke RKA, Zschiesche E: A randomized, blinded, controlled and multi-centered field study comparing the efficacy and safety of BravectoTM (fluralaner) against FrontlineTM (fipronil) in flea- and tick-infested dogs. Parasit Vectors. 2014, 7: 83.
2 Welling PG: Effects of food on drug absorption. Annu Rev Nutr. 1996, 16: 383-415.
3 Singh BN: Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999, 37: 213-255.
4 Schmidt LE, Dalhoff K: Food-drug interactions. Drugs. 2002, 62: 1481-1502.
5 FDA: Guidance for Industry, Bioanalytical Method Validation. 2001, Rockville: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM)
Related articles And Qustion
See also
Lastest Price from Fluralaner manufacturers

US $0.00-0.00/g2024-12-24
- CAS:
- 864731-61-3
- Min. Order:
- 1g
- Purity:
- 99.99%
- Supply Ability:
- 20 tons

US $0.00/kg2024-12-17
- CAS:
- 864731-61-3
- Min. Order:
- 10kg
- Purity:
- 99% purity
- Supply Ability:
- 1000 kg