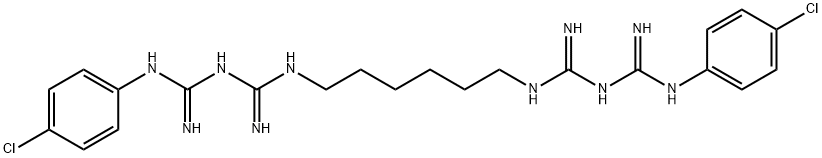
The antibacterial properties of chlorhexidine
Introduction
Chlorhexidine remains the gold standard to which other antiplaque and gingivitis agents are compared. Its effectiveness can be attributed to it bactericidal and bacteriostatic effects and its substantivity within the oral cavity. While other agents may possess one or more of these properties, few possess all three and perform so well. The antimicrobial properties of chlorhexidine are attributed to it di-cationic structure, and it is this same property that is the basis of its most common side effect, extrinsic tooth staining. By understanding the biochemical properties of chlorhexidine, an appreciation for the efficacy and use of chlorhexidine can be developed. It is only in such a way that the efficacy can be maximized and the side effects minimized, allowing chlorhexidine to remain the gold standard [1].
Chlorhexidine is an important medical, dental and pharmaceutical antiseptic, disinfectant and preservative. It is bactericida and fungicidalsy but does not kill bacterial spores or mycobacteria, although it inhibits growth. It has a low order of activity against viruses, but high concentrations are effective in killing cysts of Acanthamoeba spp., organisms of potential clinical significance to the wearers of contact lenses. Properties Chlorhexidine is a bisbiguanide which is available as the acetate (diacetate), hydrochloride and gluconate salts. These are stable in solution and can be autoclaved although small amounts of chloroaniline are released.” As a cationic agent, chlorhexidine is incompatible with anionic surfactants and its antimicrobial activity is reduced in the presence of non-ionic surface-active agents. Activity is also reduced or abolished by phospholipids (a factor of significance in neutralizing chlorhexidine activity during the performance of biocidal tests) and by organic matter including serum. Some of these aspects have been well documented in the recent comprehensive paper of Nicoletti et al.” They also point out that the efficacy of chlorhexidine is influenced by formulation components and by the composition of the culture medium in which minimum inhibitory concentrations (MICs) are determined [2].
After 20 years of use by the dental profession, chlorhexidine is recognized as the gold standard against which other antiplaque and gingivitis agents are measured. Chlorhexidine's antiplaque effect is a result of the dicationic nature of the chlorhexidine molecule, which affords the agent the property of persistence of antimicrobial effect at the tooth surface, through both bactericidal and bacteriostatic effects. Although other antiplaque agents may show either purely immediate effect, or limited persistence, the degree of chlorhexidine's persistence of effect at the tooth surface is the basis of its clinical efficacy. Similarly, the cationic nature of the chlorhexidine molecule is the basis of the most common side effect associated with the use of the agent--extrinsic tooth staining. Such tooth staining seems to be the result of a local precipitation reaction between tooth-bound chlorhexidine and chromogens found within foodstuffs and beverages. The cationic nature of the chlorhexidine molecule also means that the activity of the agent is rapidly reduced in the presence of anionic agents, specifically those found within certain types of toothpaste; thus care is required when using normal toothbrushing alongside chlorhexidine. By understanding how the chemical properties of the chlorhexidine molecule can explain the plethora of clinical efficacy and safety data, the use of chlorhexidine can be optimally aimed towards the patient groups who would most benefit from the superior therapeutic effect of the agent. Specifically, chlorhexidine would seem to be of most value to patients in whom the ability to perform adequate oral hygiene procedures has been compromised. In these individuals the delivery of the correct dose of chlorhexidine to the tooth surface can be optimized through the judicial use of the several different chlorhexidine formulations now available. Thus, by understanding the properties and limitations of the chlorhexidine molecule, the dental profession can ensure that the efficacy of the agent is maximized, and the side effects associated with the agent are minimized, allowing chlorhexidine to rightly remain the gold standard against which other antiplaque agents are measured.
Some antibacterial properties of chlorhexidine
The use of the antiseptic chlorhexidine as a therapeutic or prophylactic drug in dental or periodontal hygiene is a departure from the usual fields of use for this agent. Therefore, a review of some of its antibacterial properties is desirable.
The bacteriostatic spectrum of chlorhexidine was determined: there was a wide spectrum of activity with gram-positive cocci being especially sensitive (MIC 0.19 to 2.0 μg/ml). Exposure of suspensions of various bacterial species to chlorhexidine (0.02%) for 10 minutes at room temperature, reduced the viable organisms by about 99.99% in most instances. Addition of serum to the test system reduced the bactericidal action markedly. A study with Streptococcus mutans revealed that the presence of sucrose (5%) in the growth-medium used for preparing bacterial suspensions, profoundly influenced the bactericidal activity. This is presumed to be the result off adsorption of chlorhexidine to extracellular polysaccharides.
Attempts to select Escherichia coli and oral streptococci resistant to chlorhexidine by serial passage in subinhibitory concentrations in vitro, indicated that mutants were rare. In contrast, mutants of E. coli resistant to ampicillin or streptomycin were selected with ease. Predictions of what might happen under clinical conditions (where oral organisms might be exposed to sublethal concentrations for very long periods of time) cannot be made from such laboratory experiments. No changes were observed in the susceptibility of faecal organisms of hamsters given chlorhexidine (50 mg/kg) daily by gastric catheter for 28 days but 100 mg/kg rapidly caused a lethal enteritis which was assumed to be the result of gross disturbances of the alimentary microflora.
A small study in three human volunteers is reported. Use of a mouthrinse (twice a day for up to seven weeks) containing chlorhexidine digluconate (0.2%) resulted in a slight but transient change in susceptibility of salivary organisms to the bacteriostatic action of the agent. Whether resistance will prove to be an obstacle to the use of chlorhexidine in prophylaxis, in the mouth can be decided only with more clinical experience.
Reference
1 Moshrefi A. Chlorhexidine[C]//The Journal of the Western Society of Periodontology/periodontal Abstracts. 2002, 50(1): 5-9.
2 A.D.Russell, M.J.Day. Antibacterial activity of chlorhexidine. Journal of Hospital Infection199325(4): 229-238
3 Jones C G. Chlorhexidine: is it still the gold standard?[J]. Periodontology 2000, 1997, 15: 55-62.
4 Hennessey T D. Some antibacterial properties of chlorhexidine[J]. Journal of periodontal Research, 1973, 8: 61-67.
You may like
Related articles And Qustion
Lastest Price from Chlorhexidine manufacturers

US $1.00/kg2025-04-21
- CAS:
- 55-56-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $120.00/kg2025-04-15
- CAS:
- 55-56-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10ton