Fluralaner (INN) is a systemic insecticide and acaricide that is administered orally. The U.S. Food and Drug Administration (FDA) approved it under the trade name Bravecto for flea treatment in dogs in May 2014. The EU approved the drug in February 2014. Australia approved it for the treatment and prevention of ticks and fleas on dogs in January 2015.
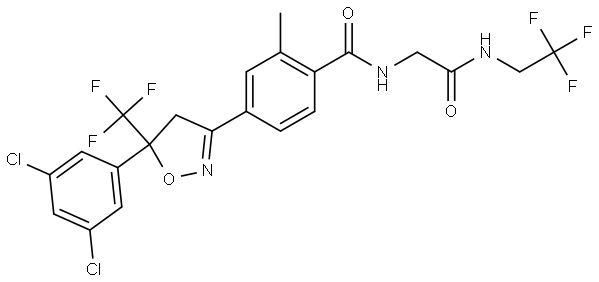
Fluralaner(864731-61-3) is a novel systemically administered insecticidal and acaricidal compound that provides long-acting efficacy after oral administration to dogs. Fluralaner belongs to a new class of compounds, the isoxazolines. A field study has shown that a single fluralaner dose administered orally to dogs provides at least twelve weeks of flea- and tick-control. The long duration of activity offers a more convenient treatment over monthly flea and tick control treatments with a potential compliance advantage, reducing the risk of vector-transmitted diseases.
Fluralaner is an insecticide and acaricide used to treat Sarcoptes scabiei var. canis infestation in dogs. Flea treatment. A possible anti-malarial found to kill disease-spreading mosquitoes when they bite treated people.
Following oral administration, fluralaner is readily absorbed reaching maximum plasma concentrations within 1 day. Food enhances the absorption. Fluralaner is systemically distributed and reaches the highest concentrations in fat, followed by liver, kidney and muscle. The prolonged persistence and slow elimination from plasma (t1/2 = 12 days) and the lack of extensive metabolism provide effective concentrations of fluralaner for the duration of the inter-dosing interval. Individual variation in Cmax and t1/2 was observed. The major route of elimination is the excretion of unchanged fluralaner in faeces (~90% of the dose). Renal clearance is the minor route of elimination.
Most pets have very few side effects from fluralaner, provided it is given according to label recommendations and at the prescribed interval (or for off-label use, according to your veterinarian's directions). Side effects may include vomiting, diarrhea, decreased appetite, or flaky skin.
vcahospitals.com
No adverse reactions were observed following oral administration to puppies aged 8–9 weeks and weighing 2.0–3.6 kg treated with overdoses of up to 5 times the maximum recommended dose (56 mg, 168 mg and 280 mg fluralaner/kg bodyweight) on three occasions at shorter intervals than recommended (8-week intervals).
There were no findings on reproductive performance and no findings of concern on offspring viability when fluralaner was administered orally to Beagle dogs at overdoses of up to 3 times the maximum recommended dose (up to 168 mg/kg bodyweight of fluralaner).
The veterinary medicinal product was well tolerated in Collies with a deficient multidrug-resistanceprotein 1 (MDR1 -/-) following single oral administration at 3 times the recommended dose (168 mg/kg bodyweight). No treatment-related clinical signs were observed.
Fluralaner is highly bound to plasma proteins and might compete with other highly bound drugs such as non-steroidal anti-inflammatory drugs (NSAIDs) and the cumarin derivative warfarin. Incubation of fluralaner in the presence of carprofen or warfarin in dog plasma at maximum expected plasma concentrations did not reduce the protein binding of fluralaner, carprofen or warfarin.
During clinical field testing, no interactions between Bravecto chewable tablets for dogs and routinely used veterinary medicinal products were observed.
Fluralaner is an inhibitor of the arthropod nervous system. It inhibits γ-aminobutyric acid (GABA)-gated chloride channels (GABAA receptors) and L-glutamate-gated chloride channels (GluCls). Potency of fluralaner is comparable to fipronil (a related GABA-antagonist insecticide and acaricide).