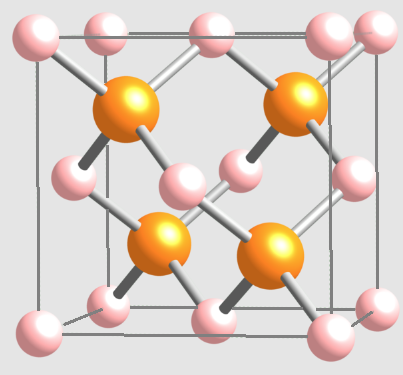
The crystal strucure of Aluminum arsenide
Compounds containing Group IIIA and Group VA elements include GaP and GaAs. Such compounds are isoelectronic with silicon; like silicon, they behave as useful semiconductors. Gallium arsenide is used in the manufacture of light-emitting diodes (LEDs). Aluminum arsenide is also used in electronic devices such as transistors, thermistors, and rectifiers. In general, compounds of the Group IIIA and VA elements are prepared by heating a mixture of the two elements.
Aluminum arsenide (AlAs) is instrumental in various high-tech applications ranging from high-speed electronics, optoelectronics, and integrated circuits to solar energy and quantum technology. The similar lattice constants of AlAs and GaAs minimize strain in layers, enabling the development of high-performance devices like HEMTs and quantum well devices. AlAs's wider band gap compared to GaAs enhances its suitability for high-temperature operations, high-power applications, and high-frequency devices.
Aluminum arsenide (AlAs) is a member of the group III-V semiconductors. At room temperature, AIAs have a cubic crystal structure and a direct band gap of about 2.16 eV. AIAs share a similar lattice constant with gallium arsenide (GaAs) and aluminum gallium arsenide, which enables it to form a superlattice with GaAs, enhancing its semiconductor properties. The wider band gap of AlAs (compared to GaAs) gives it some especially desirable qualities.
You may like
Lastest Price from ALUMINUM ARSENIDE manufacturers

US $15.00-10.00/KG2021-08-12
- CAS:
- 22831-42-1
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton