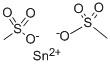
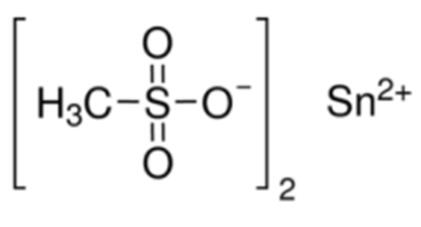
Stannous Methanesulfonate: Electrolytic Preparation and Applications in Proteomics
Description
Stannous methanesulfonate is a kind of important organo-tin compound, be widely used in organic synthesis and the eleetrotinplate technology, for example in the eleetrotinplate technology as tinned main salt and additive, substituting with fluoroborate is the eleetrotinplate technology of main salt.

Property
Tin methanesulfonate occurs in a solution with a color varying from colorless to pale yellow. It is soluble in water and contains 300 g/l of tin.
Electrolytic preparation
Electrolysis method was used to prepared stannous methanesulfonate by using tin plate as the anode and graphite as the cathode in the methylsulfonic acid solution. Based on the uniform design experimental program, the multivariate nonlinear regression equation, which described quantitatively the inherent law of experiment, was established. The optimal conditions of synthesis were determined by data mining technology. The conversion rate was 97% when in optical conditions. From the results, it was shown that the influence degree of each factor is as follows: current density > electrolyte concentration > stirring speed > reaction temperature. 1
Applications in proteomics
A primary application of stannous methanesulfonate in proteomics lies in its capacity to reduce disulfide bonds within proteins. This action aids in the denaturation and digestion of proteins into smaller peptides. By disrupting disulfide bonds, stannous methanesulfonate facilitates the unfolding of intricate protein structures, rendering them more amenable to enzymatic cleavage and subsequent analysis.2
1. Sun J, Zhang XW, Meng JH. Electrolytic Preparation of Stannous Methanesulfonate Using Uniform Design Method. Advanced Materials Research. 2011; 15(1): 602-605.
2. Yang KK, Mahmoudian M, Ebadi M, Koay HL, Basirun W. Diffusion Coefficient of Tin(II) Methanesulfonate in Ionic Liquid and Methane Sulfonic Acid (MSA) Solvent. Metallurgical and Materials Transactions B. 2011; 19(1): 1274-1279.
References:
[1] JIE SUN J M Xingwei Zhang. Electrolytic Preparation of Stannous Methanesulfonate Using Uniform Design Method[J]. Advanced Materials Research, 2011, 15 1: 119-138. DOI:10.4028/www.scientific.net/AMR.197-198.602.[2] KOK KEE YANG. Diffusion Coefficient of Tin(II) Methanesulfonate in Ionic Liquid and Methane Sulfonic Acid (MSA) Solvent[J]. Metallurgical and Materials Transactions B, 2011, 19 1: 7-8. DOI:10.1007/S11663-011-9560-Z.
You may like
Related articles And Qustion
See also
Lastest Price from Stannous methanesulfonate manufacturers
US $1.00/kg2025-04-21
- CAS:
- 53408-94-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $10.00/kg2025-04-15
- CAS:
- 53408-94-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton