The brief introduction of Tellurium
Description
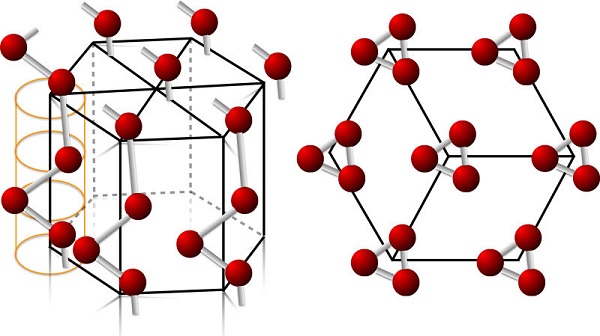
With an abundance in the Earth's crust similar to that of Pt (about 1 μg/kg), tellurium is one of the rarest stable solid elements. In comparison, even the rarest of the stable lanthanides have crustal abundances of 500 μg/kg. Tellurium is a member of the chalcogens and is chemically related to other members of the group such as selenium and sulfur. Tellurium has two forms: crystalline and amorphous. Crystalline Te is the common silvery-white, lustrous form. It is a brittle and easily pulverized metalloid. Amorphous Te appears as a black-brown powder and forms when precipitated out of tellurous acid or telluric acid (Te(OH)6).
Tellurium is sometimes found in its native (i.e., elemental) form, but is more often found as the tellurides of gold such as calaverite and krennerite (two different polymorphs of AuTe2), petzite (Ag3AuTe2), and sylvanite (AgAuTe4). Gold itself is usually found on its own, but when found as a chemical compound, it is most frequently together with tellurium. Although tellurium is found with gold more often than in its native form, it is observed to occur even more often as tellurides in combination with more common metals.
Chemical Properties
Its atomic number is 52 and its electrons are arranged in an electronic configuration of [Kr]4d105s2 5p4. There are six electrons in the outer shell, but as with its post-transition neighbors, the inert pair effect makes an oxidation state that is less than the valence especially stable. Compounds of tellurium are thus seen in the +4 oxidation state. Nevertheless, compounds with oxidation states of -2, +2, and +6 are common. The oxidation state of -2 is found when Te is combined with strongly electropositive metals such as group 1, group 2, and lanthanides. The +2, +4, and +6 oxidation states are found when Te is combined with electronegative elements, Cl, O, and F. It combines with most elements, although less readily than O and S. For instance, it can corrode iron, copper, and steel but only in its molten state.
Preparation
The primary tellurium source comes from anode sludges from the electrolytic refining of blister copper. It is also a component of dust from blast furnace refining of lead. Processing of 1000 tons of copper ore typically produces 1 kg of tellurium. The anode sludges have the selenides and tellurides of the noble metals present in compounds with the general formula M2Se or M2Te (M=Cu, Ag, Au). At temperatures of ~500 ℃, the anode sludges are roasted with sodium carbonate (Na2CO3) under air. In this reaction, the metal ions are reduced to the metals, while at the same time, the telluride is converted to sodium tellurite.
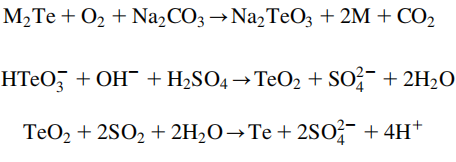
Tellurites can be leached from the mixture with water and are usually present as hydrotellurites HTeO3- in solution. Selenites are also formed in this reaction, but they can be isolated by the addition of sulfuric acid. This addition causes the hydrotellurites to be converted into the insoluble tellurium dioxide (TeO2) while the selenites stay in solution.
Finally, the metal is obtained from the oxide through reduction either by electrolysis or by reacting the tellurium dioxide with sulfur dioxide in sulfuric acid.
Minerals

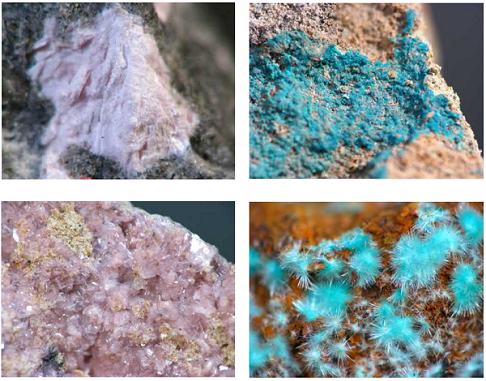
About 150 minerals are known to contain tellurium in their crystal structures. Tellurium (Te) occurs as a native element. The largest group is found in the sulfide class with 76 different minerals. Two halides, kolarite (PbTeCl2) and radhakrishnaite (PbTe3(Cl,S)2), have Te in their structure. Nearly 50 minerals in the oxide class are known to contain Te, for example, emmonsite, fairbankite (Pb(TeO3)), spiroffite, and tellurite (TeO2). Twelve minerals are found in the sulfate class, which includes the tellurates, for example, montanite (Bi2(TeO6)· 2H2O) and utahite (Cu5Zn3(TeO4)4(OH)8·7H2O). Three phosphate class minerals, cheremnykhite (Pb3Zn3(VO4)2(TeO6)), dugganite (Pb3Zn3(AsO4)2(TeO6)), and kuksite (Pb3Zn3(PO4)2(TeO6)), are known to contain Te. The only known silicate is burckhardite (Pb2(Fe3+Te6+)[AlSi3O8]O6).
You may like
Related articles And Qustion
See also
Lastest Price from Tellurium manufacturers

US $1.00/g2019-07-06
- CAS:
- 13494-80-9
- Min. Order:
- 50 g
- Purity:
- 99.9%
- Supply Ability:
- 20kg