Neodymium:Description, Minerals, and Reactions
Description
Neodymium (Nd) belongs to the lanthanide series and is a rare earth element (REE). It is a hard, slightly malleable, silvery metal. When oxidized, neodymium reacts rapidly to form pink, purple/blue, and yellow compounds in the +2, +3, and +4 oxidation states, respectively. Metallic neodymium has a bright, silvery metallic luster, but as one of the more reactive lanthanide rare-earth metals, it rapidly oxidizes in air. The oxide layer that forms then peels off, exposing the metal to further oxidation. Consequently, a centimeter-sized piece of neodymium completely oxidizes in less than a year. Neodymium commonly exists in two allotropic forms, with a transformation from a double hexagonal close-packed (dhcp) to a body-centered cubic (bcc) structure occurring at approximately 863 ℃.
Although it belongs to the rare-earth metals, neodymium is not rare at all. Its abundance in the Earth’s crust is about 38 ppm, the second highest among REEs, following cerium. Neodymium is not found in nature as a free element, but instead it occurs in ore minerals.
Minerals
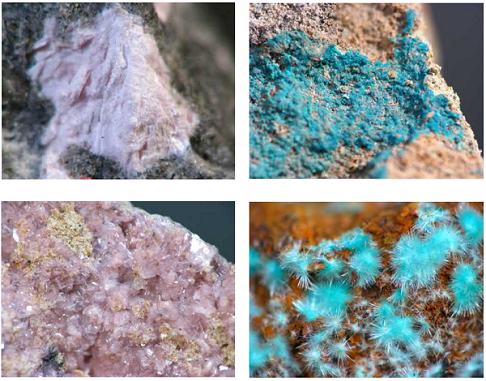
Neodymium typically constitutes between 10% and 18% of the rare-earth content of commercial ore deposits of the light REE minerals bastnäsite and monazite. With neodymium compounds being the most strongly colored of the trivalent lanthanides, it can sporadically dominate the coloration of rare-earth minerals when competing chromophores are absent. It usually gives a pink colouration. Great examples of this include monazite crystals from the tin deposits in Llallagua, Bolivia; ancylite (Sr(Ce, La)(CO3)2(OH)·H2O) from Mont Saint-Hilaire, Quebec, Canada; or lanthanite from the Saucon Valley, Pennsylvania, United States. As with neodymium glasses, such minerals change colors under different lighting conditions. The absorption bands of neodymium interact with the visible emission spectrum of mercury vapour, with the unfiltered shortwave UV light causing neodymium-containing minerals to reflect a characteristic green colour. This can be seen with monazite-containing sands or bastnäsite-containing ore.
Only 37 minerals are known with neodymium in their chemical composition. Chukhrovite-(Nd) (Ca3(Nd, Y)Al2(SO4)F13· 12H2O) is the only known halide.
Four oxides are known with Nd, including aeschynite-(Nd) ((Nd,Ln,Ca)(Ti,Nb)2(O,OH)6) and fergusite-(Ce)/fergusonite-(Nd) ((Nd,Ce)NbO4).
The carbonates are represented by 12 different minerals, such as bastnäsite-(Nd) (Nd(CO3)F), kozoite-(Nd) ((Nd,La,Sm,Pr)(CO3)(OH)), schuilingite-(Nd) (PbCu(Nd,Gd,Sm,Y)(CO3)3(OH)·1.5H2O), and synchysite-(Nd) (CaNd(CO3)2F).
The same number of minerals is found in the phosphate class, for example, agardite-(Nd) (NdCu6(AsO4)3(OH)6·3H2O), florencite-(Nd) (NdAl3(PO4)2(OH)6), monazite-(Nd) (Nd(PO4)), rhabdophane-(Nd) (Nd(PO4)·H2O), and wakefieldite (Nd(VO4)).
The silicate class contains eight minerals with Nd, for example, allanite-(Nd) ({CaNd}{Al2Fe21}(Si2O7)(SiO4)O(OH)), askagenite-(Nd) ({Mn21Nd}{Al2Fe31}(Si2O7)(SiO4)O2), and swamboite-(Nd) (Nd0.333(UO2)(SiO3OH)2.5).
Reactions
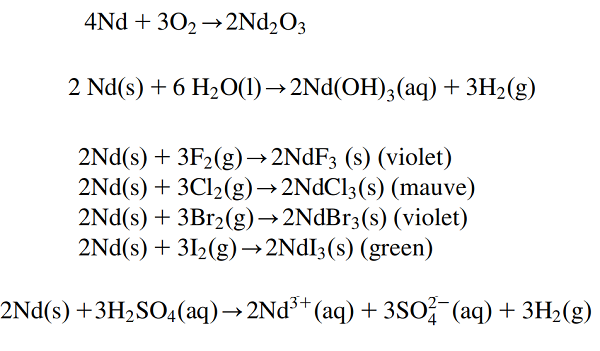
a. Neodymium metal tarnishes slowly in air and burns readily at about 150 ℃ to form neodymium(III) oxide. It forms very light grayish-blue hexagonal crystals.
b. It is a fairly electropositive element, reacting slowly with cold water but rather rapidly with hot water to form neodymium(III) hydroxide.
c. Neodymium metal reacts vigorously with all the halogens.
d. It dissolves readily in dilute sulfuric acid to form solutions containing the lilac Nd(III) ion. These exist as [Nd(H2O)9]3+ complexes.


