The brief introduction of Eptifibatide
Introduction

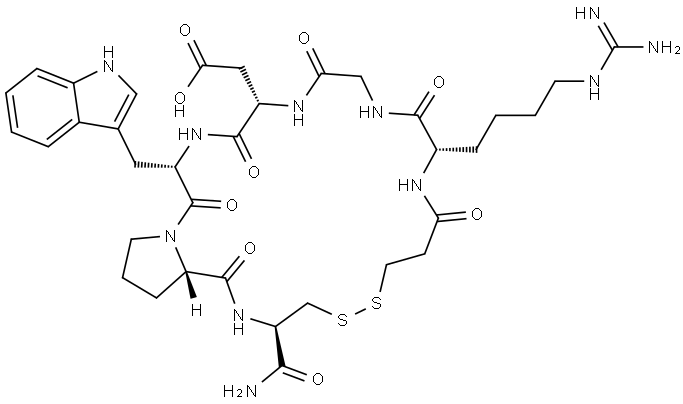
Eptifibatide (Integrilin), a disulfide-linked cyclic peptide, is a short-acting and reversible inhibitor of platelet aggregation. Eptifibatide contains a KGD (lysine–glycine–aspartic acid) sequence similar to the snake venom barbourin. This sequence is purported to selectively block the platelet glycoprotein IIb–IIIa complex without affecting the functions of other integrins. The potential benefits of eptifibatide include its ability to bind reversibly and its advantage in treating patients at high risk for bleeding. Eptifibatide has been evaluated in acute coronary syndromes, extracorporeal bypasses, and normal human volunteers. A recently conducted phase III angioplasty trial with eptifibatide demonstrated a favourable trend for a reduction in the incidence of death, myocardial infarction, and urgent intervention in patients treated with eptifibatide.
Indication
It is a glycoprotein IIb/IIIa class platelet inhibitor drug that reduces ischemic cardiac events in specific patient populations. The FDA has approved this medication for the medical management of unstable angina and non-ST elevation myocardial infarction (NSTEMI)[1]. The FDA also approved eptifibatide for patients undergoing percutaneous intervention (PCI), including intracoronary stenting. The IMPACT-II trial proved that eptifibatide use with heparin and aspirin reduces ischemic events following a PCI, especially in individuals with unstable angina.
Clinical research
Eptifibatide is a small cyclic heptapeptide molecule which competitively and reversibly binds to the GPIIb-IIIa receptor. Compared to abciximab, this drug has a lower affinity to the GPIIb-IIIa receptor (although with higher specificity), resulting in easier ligand-receptor dissociation[2]. Due to this, the plasma and biological half-life of eptifibatide is much shorter and is estimated to be around 2 h after drug discontinuation. Effects of eptifibatide were overestimated by in vivo studies where calcium chelators were simultaneously used. This resulted in suboptimal dosing of eptifibatide during the early clinical trials. The Integrilin to minimize platelet aggregation and coronary thrombosis-II (IMPACT-II) trial showed that a loading dose of 135 μg/kg followed by a maintenance infusion of 0.5–0.75 μg/kg/min resulted in inadequate inhibition of platelet aggregation and hence failed to reveal any clinical benefit. Alteration of the dosing regimen to 180 μg/kg as the loading dose results in sufficient platelet inhibition within 15 min. However, after a single loading dose followed by maintenance infusion, the platelet inhibition continues to decline over the 4–6 h needed to achieve a steady plasma state. For this reason and based on the results of the ESPRIT trial, a double bolus regimen (two 180 μg/kg boluses 10 min apart followed by an infusion of 2 μg/kg/min for 18–24 h) is often adopted in the percutaneous coronary intervention (PCI) setting. Systemic clearance of the drug is accomplished by the kidneys, and hence, patients with creatinine clearance < 50 mL/min require dose adjustments. Eptifibatide is currently contraindicated in patients on hemodialysis, although it is theorized that this drug may be cleared by hemodialysis as it is not extensively bound by plasma proteins. Similar to abciximab, anaphylaxis and thrombocytopenia using eptifibatide have been uncommonly reported. Although bleeding remains a common side effect, studies have shown the incidence of TIMI significant bleeding rates to be similar to those with a placebo.
Side effects
Common side effects of eptifibatide may include bleeding or feeling lightheaded.
An allergic reaction: hives; difficulty breathing; swelling of your face, lips, tongue, or throat.
A lightheaded feeling, like you might pass out; any bleeding that will not stop; bleeding around your IV or catheter, or in any place where your skin has been punctured with a needle; red or pink urine; or signs of stomach bleeding--bloody or tarry stools, coughing up blood or vomit that looks like coffee grounds.
The risk of bleeding may be higher in older adults.
References
[1] “Eptifibatide.” Reactions Weekly 83 1 (2021): 156–156.
[2] Bavry, A. “αIIbβ3 (GPIIb-IIIa) Antagonists.”Platelets (Fourth Edition) (2019): 957-971.
You may like
Related articles And Qustion
Lastest Price from Eptifibatide manufacturers

US $1.00/g2025-09-22
- CAS:
- 188627-80-7
- Min. Order:
- 10g
- Purity:
- 99
- Supply Ability:
- 999

US $1000.00-1000.00/g2025-09-19
- CAS:
- 188627-80-7
- Min. Order:
- 1g
- Purity:
- >=99% (HPLC), USP43
- Supply Ability:
- 10000000g