The application of vinylene carbonate in Li-ion batteries
Introduction
Vinylene carbonate is a monomer used for producing important technical products, e.g., polyvinylene carbonate, polyvinylene glycol, and polyvinylene acetal. From polyvinylene carbonate and its derivatives, one can obtain high[1] strength fibers, films and organic glass resistant to destructive effects, prophylactic drugs against ionizing radiation, and selective sorbents of boron and other rare elements
Vinylene carbonate, as well as the majority of 1,2substituted ethylene compounds, has a low activity in the reactions of copolymerization. The structure of the vinylene carbonate radical determines its tendency toward reactions of chain transfer to a solvent or an impurity; this leads to a significant decrease in the copolymerization rate and the molecular weight of a copolymer produced[1].

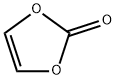
Picture 1 Vinylene carbonate liquid
Properties
The vinylene carbonate (VC) is a cyclic, reactive, unsaturated carbonate ester. The role of VC as an additive was studied and the solid electrolyte interfacial layer resulting from its reduction was characterized by infrared spectroscopy. The best use of VC is, as a component of surface coatings. Although there are reports on the additive characteristics of VC, as yet a scanty of study on the electrical properties of thin films obtained from VC has been appeared in the literature. The vinylene compounds have applications in the electrical and optical devices. So the investigations of the structural and electrical properties of thin films grown from VC are of necessity for its application in various devices. From this point of view, the studies of the structural and electrical properties of plasma polymerized vinylene carbonate (PPVC) thin films have been undertaken. In this paper, the structural characteristics by Fourier transform infrared (FTIR) analysis and the current density[1]voltage (J–V) characteristics at different temperatures of the PPVC are presented.
Application
Vinylene carbonate (VC) was tested as an additive to electrolyte solutions for Li-ion batteries. For the model electrodes, synthetic graphite was chosen as the anode material, while LiMn2O4 spinel and LiNiO2 were chosen as the cathode materials[2]. The test solution was 1 M LiAsF6 in a 1:1 mixture of ethylene and dimethyl carbonates (EC–DMC). Cyclic voltammetry (CV), chronopotentiometry, impedance spectroscopy, electrochemical quartz crystal microbalance (EQCM), FTIR and X-ray photoelectron spectroscopies have been used in this study. It was found that VC is a reactive additive that reacts on both the anode and the cathode surfaces. The influence of this additive on the behavior of Li–graphite anodes is very positive, since it improves their cyclability, especially at elevated temperatures, and reduces the irreversible capacity. The spectroscopic studies indicate that VC polymerizes on the lithiated graphite surfaces, thus forming poly alkyl Li-carbonate species that suppress both solvent and salt anion reduction. The presence of VC in solutions reduces the impedance of the LiMn2O4 and LiNiO2 cathodes at room temperature. However, we have not yet found any pronounced impact of VC on the cycling behavior of the cathodes, either at room temperature or at elevated temperatures. Thus, VC can be considered as a desirable additive for the anode side in Li-ion batteries, one which has no adverse effect on the cathode side.
Enzyme Immobilization Based on Copolymers of Vinylene Carbonate
In this study, a series of beadlike and hydrophilic supports containing reactive cyclic carbonate groups for enzyme immobilization were prepared via reverse-phase suspension copolymerization of the aqueous solutions of vinylene carbonate (VCA), acrylamide (AA), and N,N'-methylene bisacrylamide in paraffin oil. The supports were used as a matrix for immobilization of trypsin and showed a considerable capacity to couple with trypsin and reasonable retention of activity for the immobilized trypsin, depending on the immobilization conditions, such as the content of VCA structural units, reaction time, and pH of the medium[3].
Reference
1 Rzhekhina, E.K. Heterophase polymerization of vinylene carbonate in octane. Polym. Sci. Ser. D 5, 86–88 (2012).
2 Majumder, S., Bhuiyan, A.H. DC conduction mechanism in plasma polymerized vinylene carbonate thin films prepared by glow discharge technique. Polym. Sci. Ser. A 53, 85–91 (2011).
3 Ding, L., Jiang, Y., Huang, L. et al. New supports for enzyme immobilization based on copolymers of vinylene carbonate and acrylamide. Appl Biochem Biotechnol 95, 11–21 (2001).
You may like
Related articles And Qustion
See also
Lastest Price from Vinylene carbonate manufacturers

US $1.00/kg2025-04-21
- CAS:
- 872-36-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $30.00-10.00/KG2025-04-15
- CAS:
- 872-36-6
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg