Vinylene Carbonate: Versatile Role in Organic Synthesis and its Green Preparation
General Description
Vinylene carbonate is a versatile compound in organic synthesis, acting as an oxidizing acetylene surrogate and facilitating the creation of complex cyclic structures through various reactions, notably rhodium-catalyzed annulations. Research has shown its effectiveness in transforming starting materials into valuable products, such as 4-methylquinazolines, by participating in annulation with diynes and amidines. Green methodologies for Vinylene carbonate's preparation focus on organic amines as dechlorination catalysts, yielding significant advancements in efficiency and sustainability. Recent innovations emphasize the use of blocking agents to reduce polymerization, achieving yields exceeding 95% in batch processes and over 98% in continuous methods, thus enhancing production efficiency.

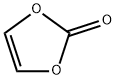
Figure 1. Vinylene carbonate
Versatile Role in Organic Synthesis
Introduction
Vinylene carbonate is a unique compound in organic chemistry, recognized for its role as an oxidizing acetylene or ethynol surrogate. Its innovative structure and properties allow vinylene carbonate to participate in various annulation reactions, facilitating the synthesis of complex cyclic frameworks. Recent research has highlighted the potential of vinylene carbonate in transforming simple starting materials into valuable products through rhodium-catalyzed reactions. This highlights the versatility of vinylene carbonate, making it a crucial tool in the synthesis of intriguing compounds such as 4-methylquinazolines. 1
Applications in Organic Synthesis
The application of vinylene carbonate extends beyond simple transformations; it offers a robust mechanism for creating diverse chemical structures. For instance, Tanaka et al. demonstrated the effectiveness of vinylene carbonate in the annulation with 1,6- and 1,7-diynes, showcasing its value as an ethynol surrogate. This groundwork has been built upon by further studies, including those by Miura et al., who utilized vinylene carbonate in the synthesis of isocoumarins and isoquinolinones, demonstrating the compound's potential in generating various heterocyclic compounds. The adaptability of vinylene carbonate in these reactions is instrumental in the field of synthetic organic chemistry, allowing chemists to create intricate molecular architectures efficiently. 1
Rhodium-Catalyzed Annulation with Amidine
In more specialized research, the integration of vinylene carbonate into rhodium-catalyzed annulation involving amidines has yielded promising results. The latest findings indicate that vinylene carbonate can act as an acylation reagent rather than merely an ethynol surrogate. This adaptation leads to the formation of 4-methylquinazolines through a formal [5+1] annulation process, achieving moderate to excellent yields. Such applications underscore the evolving role of vinylene carbonate in cutting-edge organic synthesis methodologies. By enabling novel routes to compound synthesis, vinylene carbonate establishes itself as a valuable and versatile agent, bridging the gap between traditional reagents and modern synthetic needs. 1
Green preparation
The preparation of vinylene carbonate is increasingly adopting greener methodologies, primarily focusing on the use of organic amines as dechlorination catalysts. Traditional industrial processes often utilize triethylamine as a reagent to facilitate the dechlorination of chloroethyl carbonate. This method allows for a mild reaction environment that produces vinylene carbonate alongside triethylamine hydrochloride as a byproduct. Notably, patent DE-A19955944 reported a synthesis using ethylene carbonate as a solvent at 60 degrees Celsius with a yield of 73 percent, showcasing a simplified and efficient approach. However, challenges still arise from the high melting point of ethylene carbonate, complicating subsequent separation processes. Other methods, such as those detailed in US 2009/0234141, advocate for solvent-free or minimal solvent techniques using vinylene carbonate itself as the solvent, which enhances product isolation. While this method achieves a 73 percent yield, excessive concentration of vinylene carbonate can increase polymerization, potentially lowering overall yield. 2
Enhanced Methods
Recent innovations in the synthesis of vinylene carbonate emphasize optimizing the use of blocking agents that mitigate polymerization side reactions. Several patents and studies demonstrate the incorporation of novel blocking agents alongside triethylamine to suppress unwanted polymerization effectively. Techniques such as the combination of para-quinone and 2,6-di-tert-butyl-4-methylphenol have shown promising results, leading to a significant yield of 69 percent and improved selectivity. Furthermore, the method outlined in CN 112174928A employs a multi-reactor system, achieving a remarkable yield of 96.5 percent by using dimethyl carbonate as a solvent with an optimal flow rate. Innovative approaches, such as the incorporation of organic acid blocking agents with higher proton affinity than triethylamine, can be fine-tuned to optimize the process further. By consistently achieving yields exceeding 95 percent in batch synthesis, and greater than 98 percent in continuous synthesis, these greener methodologies for vinylene carbonate synthesis ultimately contribute to more sustainable and efficient chemical production processes. 2
References:
[1] WANG C, FAN X, CHEN F, et al. Vinylene carbonate: beyond the ethyne surrogate in rhodium-catalyzed annulation with amidines toward 4-methylquinazolines?[J]. Chemical Communications, 2021, 32: Page 3827 to 3942. DOI:10.1039/D1CC00882J.[2] W JOHNSON T P. Notes- Preparation of Vinylene Carbonate[J]. Journal of Organic Chemistry, 1960, 25 6: 2A - 1072. DOI:10.1021/jo01076a606.
You may like
Related articles And Qustion
See also
Lastest Price from Vinylene carbonate manufacturers

US $1.00/kg2025-04-21
- CAS:
- 872-36-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $30.00-10.00/KG2025-04-15
- CAS:
- 872-36-6
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg