The application of potassium thioacetate
General Description
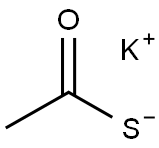
Potassium thioacetate is an organosulfur compound and a salt with the formula CH3COS−K+. This white, water-soluble solid is used as a reagent for preparing thioacetate esters and other derivatives [1]. It acts as a sulfur source in the synthesis of sulfur-containing organic compounds for the synthesis of heterocycles, polymers, transition-metal ligands, nanoparticles, bioactive compounds and macromolecular inclusion complexes [2]. It is also used for palladium mediated coupling with aryl halides and triflates leading to S-arylthioacetates and derivatives and it is also used as a reagent in the conversion of halides to thiols [3].
Transition Metal‐Catalyzed Sulfuration Reaction
Look at the transition metal‐catalyzed sulfuration reaction [4] at the following. Pd‐catalyzed preparation of S‐aryl thioacetates 2 from bromides/triflates 1 with potassium thioacetate was reported by Lai and Backes (eq 1). Under microwave irradiation at 160 °C in 1,4‐dioxane, the desired products S‐aryl thioacetates 2 could be obtained between 65 and 91%. It has been shown that many functional groups, such as N-Boc carbamate, ketone, and ethyl ester, could be well tolerated. Unfortunately, aryl chlorides are poor coupling partners. This method was applied to the preparation of novel sulfone‐containing DPP‐IV inhibitor 5. Compound 3 with the core framework of inhibitor was prepared according to the reported procedure and then coupled with potassium thioacetate under optimized reaction conditions to form key intermediate4 in 87% yield. After one-pot deacylation/ alkylation/ oxidation/ deprotection, DPP‐IV inhibitor 5 could be generated in 84% overall yield (eq 2).
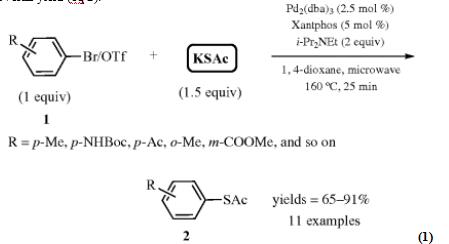
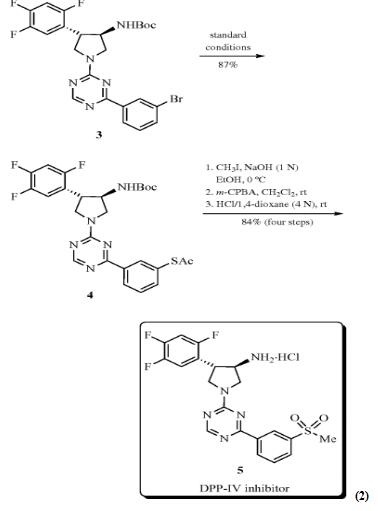
Scheme 1 Transition Metal‐Catalyzed Sulfuration
Transition Metal‐free Sulfuration Reaction
Potassium thioacetate also works in the transition metal‐free sulfuration reaction. In 2011, Wager and Daniles found that benzyl aryl thioethers 8 could be achieved by the deprotection of S‐benzyl thioacetates 7, which provided an alternative way for thioethers formation (eq 3). The authors utilized the method to achieve one‐pot reaction. Benzylbromide 6 coupled with potassium thioacetate forming S‐benzyl thioacetates 7, which could generate the desired products 8.
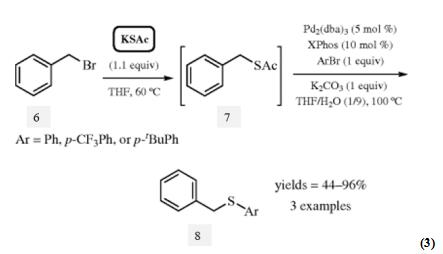
Scheme 2 Transition Metal‐Free Sulfuration
References
[1] https://en.wikipedia.org/wiki/Potassium_thioacetate
[2] https://www.selleckchem.com/products/potassium-thioacetate.html
[3] https://www.alfa.com/en/catalog/L05405/
[4] https://onlinelibrary.wiley.com/doi/10.1002/047084289X.rn01737
See also
Lastest Price from Potassium thioacetate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 10387-40-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $32.00/kg2025-04-15
- CAS:
- 10387-40-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000kg/week