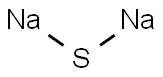The uses of sodium sulfide
Chemical Properties
Sodium sulfide is the chemical compound with the formula Na₂S, or more commonly its hydrate Na₂S·9H₂O. Both are colorless water-soluble salts that give strongly alkaline solutions. When exposed to moist air, Na₂S and its hydrates emit hydrogen sulfide, which smells like rotten eggs [1]. Sodium sulfide, anhydrous is a yellow to brick red crystalline mass or fused solid. If exposed to moist air, it absorbs moisture from the air [2], and it is liable to spontaneous heating and may cause ignition of nearby combustible material. The industrial sodium sulfide often exhibits pink, reddish brown or yellowish brown color (figure 1) for containing impurities [3].

Application of Sodium Sulfide
The aqueous water exhibits strongly alkalinity and is corrosive on copper, wood, skin, etc. It is slightly soluble in ethanol, but insoluble in ether. When being encountered strong acid, the sodium sulfide will release hydrogen sulfide. It will be subject to deliquescence in air and is easily oxidized into sodium thiosulfate. Sodium sulfide is mainly used as the raw materials of hides depilatories, pulp cooking agent, and sulfur dye, the reducing agents of dye intermediates, fabric dyeing mordant, and ore flotation agent. It can also be used as viscose fiber desulfurizer and the raw material for production of sodium hydrosulfide and sodium polysulfide [3].
As sodium sulfide is used in textile dyeing and finishing, it can be used as a bleaching agent, serving as an alternative to sodium chlorite and hydrogen peroxide. It removes yellowish stains and spots that are not removed by laundering process. Sodium sulfide can also be used as a desulfurizing and a dechlorinating agent when dealing with leather materials. In addition, it is used to obtain sulfur black dyes, which can in turn be treated and processed to obtain other color dyes.
It is primarily used in pulp and paper industry in the Kraft process of manufacturing paper. It is a component of white liquor in addition to sodium hydroxide, and white liquor plays an important role in manufacturing wood pulp. It is involved in the first stage of the Kraft process or the impregnation step where the wood chips are impregnated with the white liquor.
It is used in water treatment process to aid in the removal of metal impurities from mining-influenced-water during treatment. Sodium sulfide is also used in chemical manufacturing as a sulfonation and sulfomethylation agent. In addition to that, it is used in the photographic industry to prevent and defend a developer solution from oxidation. Sodium sulfide is also commonly used in the leather industry. It is used as one of the reagents to remove hair from hide and used in tanning of animal skin to make into leather. The unhairing process is used in the lime operation, where hide is soaked in an alkaline solution [4].
References
[1] https://en.wikipedia.org/wiki/Sodium_sulfide
[2] https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-sulfide
[3] https://www.chemicalbook.com/ChemicalProductProperty_EN_CB3429046.htm
[4] https://www.sodium-sulphide.com/product/
You may like
Related articles And Qustion
See also
Lastest Price from Sodium sulfide manufacturers

US $10.00/KG2025-04-21
- CAS:
- 1313-82-2
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/TON2024-11-07
- CAS:
- 1313-82-2
- Min. Order:
- 1TON
- Purity:
- 60%
- Supply Ability:
- 1000000