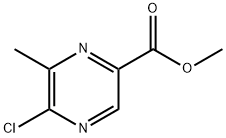
The application of methyl 5-chloro-6-methylpyrazine-2-carboxylate in physiology
The N-methyl-d-aspartate (NMDA) receptor is arguably an important signaling mechanism in the human brain and NMDA receptors play a critical role in regulating the strength of synapses, that is, in regulating synaptic plasticity. Thus, the NMDA receptor is at the molecular core of brain function, and in particular the cognitive functions of learning and memory. These facts underlie the tremendous therapeutic utility of modulating NMDA receptor function with new drugs to treat a broad range of neuropsychiatric disease and cognitive dysfunction. The NMDA receptor comprises four protein subunits: two NR1 subunits and two NR2 subunits. Methyl 5-chloro-6-methylpyrazine-2-carboxylate is the key materials to prepare the NMDA receptor [1].
The first synthesis route includes five steps to get NMDA receptor as shown in scheme 1.
The first step is the preparation of methyl 5-(bromomethyl) pyrazine-2-carboxylate. The procedure is described as below. The solution of methyl 5-methylpyrazine-2-carboxylate in acetic acid was added bromine at room temperature and the reaction mixture was heated at 80° C. for 45 min. The reaction mixture was concentrated to remove acetic acid and the residue was basified with saturated sodium bicarbonate solution and extracted with ethyl acetate. Then the organic layer was dried and concentrated, and finally the crude was purified by silica gel column chromatography using 20% ethyl acetate in hexane to afford the title compound methyl 5-(bromomethyl) pyrazine-2-carboxylate.
In the second step, the solution of methyl 5-(bromomethyl) pyrazine-2-carboxylate in chloroform was added hexamethylene tetramine and the reaction mixture was stirred at room temperature for 18 h. The solid formed was filtered and dried and the solid was suspended in methanol, concentrated hydrochloric acid was added and the reaction mixture was heated at 75° C. for 3 h. The reaction mixture was concentrated, and the residue was triturated with diethyl ether and dried to afford the title compound methyl 5-(aminomethyl) pyrazine-2-carboxylate hydrochloride as a brownish solid.
In the third step, the suspension of methyl 5-(aminomethyl) pyrazine-2-carboxylate hydrochloride in dichloromethane was added triethylamine followed by 4-fluor-3-chlorobenzenesulfonyl chloride and the reaction mixture was stirred at room temperature for 1 h. Then the reaction mixture was diluted with dichloromethane, washed with water, dried over anhydrous sodium sulfate, filtered and concentrated. Then the crude was purified by silica gel column chromatography using 40% ethyl acetate in hexane to afford the title compound methyl 5-((3-chloro-4-fluorophenylsulfonamido) methyl) pyrazine-2-carboxylate as a brownish solid. In the fourth step, to a solution of methyl 5-((3-chloro-4-fluorophenylsulfonamido) methyl) pyrazine-2-carboxylate in methanol was added 1M potassium hydroxide solution and the reaction mixture was stirred at room temperature for 1 h. The reaction mixture was concentrated, and the residue was diluted with water, acidified with 1.5 M hydrochloric acid to pH 6 and the solid separated was filtered. The precipitate was washed with ice water and dried to afford the title compound 5-((3-chloro-4-fluorophenylsulfonamido) methyl) pyrazine-2-carboxylic acid as a brownish solid.
In the fifth step, the solution of 5-((3-chloro-4-fluorophenylsulfonamido) methyl) pyrazine-2-carboxylic acid and triethylamine in dichloromethane were added 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and 1-hydroxybenzotriazole at 0° C. The reaction mixture was stirred at 0° C. for 30 min. and the solution of thiazol-5-ylmethanamine in dichloromethane was added dropwise to the reaction mixture and stirred at room temperature for 18 h. Then the reaction mixture was diluted with dichloromethane, washed with water, dried over anhydrous sodium sulfate, filtered and concentrated. Finally, the crude was purified by silica gel column chromatography using 2% methanol in dichloromethane to afford the title compound 5-[(3-chloro-4-fluorobenzene) sulfonamidomethyl]-N-(1,3-thiazol-5-ylmethyl) pyrazine-2-carboxamide as off-white solid.

Scheme 1 The synthesis of NMDA receptor
Robert described another synthesis route to prepare NMDA receptors containing GluN2A subunits shown as in scheme 2 [2].

Scheme 2 Synthesis of NMDA receptor
To get methyl substituted pyrazines (compound 7 in Scheme 2), commercially available methyl 5-chloro-6-methylpyrazine-2-carboxylate (compound 1) was saponified (KOTMS, THF) to afford acid (compound 2) which was coupled with (2-methylthiazol-5-yl) methanamine (T3P, DMF) to afford pyrazine (compound 3). Cyanide displacement (Zn(CN)2, Pd2(dba)3, Dppf, DMF) of the chloride moiety of compound 3 generated compound 4. Nitrile reduction (H2, Pd/C, MeOH) of compound 4 afforded amine compound 5. Amine (compound 5) was cleanly converted to sulfonamides compound 6 in scheme 2. Methylation of sulfonamide (compound 6, NaH, MeI) provided compound 7.
Reference
[1] Anderson, David R. and Volkmann, Robert A., Preparation of sulfonamidomethylpyridinecaboxamide derivatives and analogs for use as negative modulators of NR2A, WO2015048503.
[2] Robert A. Volkmann etc., MPX-004 and MPX-007: New Pharmacological Tools to Study the Physiology of NMDA Receptors Containing the GluN2A Subunit, PLOS ONE, February 1, 2016, 1-20
Related articles And Qustion
See also
Lastest Price from Methyl 5-chloro-6-methylpyrazine-2-carboxylate manufacturers

US $0.00-0.00/KG2020-02-26
- CAS:
- 77168-85-5
- Min. Order:
- 10g
- Purity:
- 99.0%+
- Supply Ability:
- 100 tons