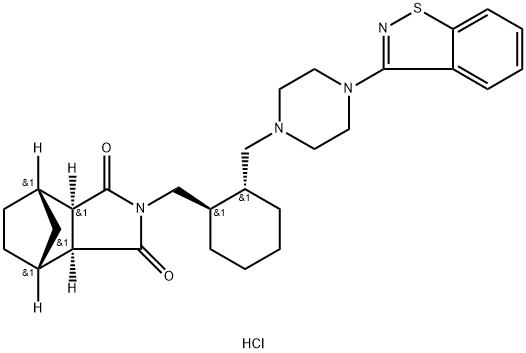
The application of Lurasidone hydrochloride
General description
The lurasidone hydrochloride, with the CAS No: 367514-88-3, is also known as Lurasidonhydrochloride. This chemical’s molecular formula is C28H36N4O2S and molecular weight is 492.684. It is an atypical antipsychotic drug used to treat schizophrenics. Compared with other existing antipsychotic drugs, lurasidone has a unique chemical structure, has affinity for dopamine D2, 5-hydroxytryptamine (5-HT2A), 5-hydroxytryptamine (5-HT7) receptors, and plays an antagonistic role in these receptors. In addition, lurasidone has partial excitatory effect on 5-hydroxytryptamine (5-HT1A) receptor, and has no obvious affinity for histamine H1 or muscarine M1 receptor.
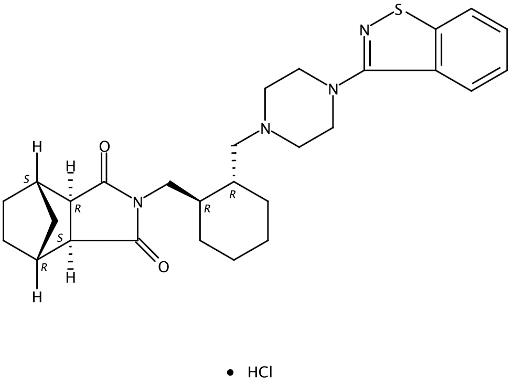
Figure 1 The molecular formula of lurasidone hydrochloride
Application
Lurasidone hydrochloride, a benzisothiazol derivative, is a second-generation (atypical) antipsychotic agent that has received regulatory approval for the treatment of schizophrenia in the US, Canada, the EU, Switzerland, and Australia, and also for bipolar depression in the US and Canada. In addition to its principal antagonist activity at dopamine D 2 and serotonin 5-HT2A receptors, lurasidone has distinctive 5-HT7 antagonistic activity, and displays partial agonism at 5-HT1A receptors, as well as modest antagonism at noradrenergic α2A and α2C receptors. Lurasidone is devoid of antihistaminic and anticholinergic activities. It is administered once daily within the range of 40–160 mg/day for schizophrenia and 20–120 mg/day for bipolar depression, and its pharmacokinetic pro?le requires administration with food. In adult healthy subjects and patients, a 40 mg dose results in peak plasma concentrations in 1–3 h, a mean elimination half-life of 18 h (mostly eliminated in the feces), and apparent volume of distribution of 6173 L; it is approximately 99 % bound to serum plasma proteins. Lurasidone’s pharmacokinetics are approximately dose proportional in healthy adults and clinical populations within the approved dosing range, and this was also found in a clinical study of children and adolescents. Lurasidone is principally metabolized by cytochrome P450 (CYP) 3A4 with minor metabolites and should not be coadministered with strong CYP3A4 inducers or inhibitors. Lurasidone does not signi?cantly inhibit or induce CYP450 hepatic enzymes[1].
Pharmacodynamics
Lurasidone hydrochloride pharmacokinetics are dose-proportional at dosages of 20–160 mg/day, with steady-state serum concentrations reached within 7 days [2]. An estimated 9–19 % of an orally administered dose is absorbed. Absorption is rapid, with serum maximum concentrations (Cmax) achieved in 1–3 h. After administration with food (irrespective of fat content), lurasidone hydrochloride Cmax and the area under the concentration–time curve (AUC) were increased approximately three- and two-fold, respectively, compared with administration after fasting. In the USA, it is recommended that lurasidone hydrochloride should be taken with a meal of at least 350 calories (i.e. a small meal or larger) [2]. The lurasidone hydrochloride mean apparent volume of distribution was 6173 L after a 40 mg dose, indicating wide distribution in the body [2]. Lurasidone hydrochloride was 99 % bound to serum proteins [2]. Lurasidone hydrochloride is metabolized by hepatic cytochrome P450 (CYP) 3A4 enzymes and does not appear to be a substrate of other clinically important CYP enzymes [2]. Lurasidone hydrochloride is catabolized through oxidative N-dealkylation, hydroxylation of a norbornane ring and S-oxidation, forming two active metabolites (ID-14283 and ID-14326), along with major non-active metabolites [2]. Lurasidone hydrochloride is chie?y excreted via the gastrointestinal tract, as after a single radiolabelled dose of lurasidone hydrochloride, 80 % of the radioactivity was detected in the faeces and 9 % in the urine [2]. The mean apparent clearance of lurasidone after a 40 mg dose was 3902 mL/min and the mean elimination half-life was 18 h [2].
Mechanism of action
The mechanism of action of lurasidone hydrochloride is poorly understood, but combined antagonism of D2 and 5-HT2A receptors is thought to be important to its antipsychotic and antidepressant effects [2, 3, 4]. Relative to some other antipsychotics (haloperidol, clozapine, olanzapine and risperidone), lurasidone hydrochloride has higher af?nity for D2, 5-HT2A and 5-HT7 receptors, with the exception of risperidone, which has a higher af?nity for 5-HT2A receptors than lurasidone hydrochloride [4]. Agonist activity at 5-HT1A receptors has been associated with anxiolytic and antidepressant-like activity in humans and rodents, and it is possible that antagonism of 5-HT7 receptors underpins positive effects on learning, memory and affective symptoms [4]. Lurasidone hydrochloride binding to α2C-adrenergic receptors may also be relevant to its antidepressant effects [3, 4]. Given this pharmacological pro?le, it is anticipated that lurasidone hydrochloride will have both antipsychotic and antidepressant effects [3, 4].
Safety and Tolerability
Lurasidone hydrochloride was generally well tolerated in the PREVAIL 1, 2 and 3 clinical trials. In PREVAIL 2 (monotherapy), 62, 65 and 57 % of lurasidone hydrochloride 20–60, 80–120 mg/day and placebo recipients, respectively, had adverse events (\10 % of events were rated severe); in the corresponding groups, 1, 1 and 0 % had a serious adverse event (SAE) and 7, 6 and 6 % withdrew from treatment because of an adverse event [5]. In PREVAIL 1 (adjunctive therapy), 64 and 58 % of lurasidone hydrochloride and placebo recipients, respectively, had an adverse event (the majority rated as mild or moderate in severity); 1 % of patients in both treatment groups had a SAE, and 6 % of lurasidone hydrochloride and 8 % of placebo recipients withdrew from treatment because of an adverse event [6]. There were no instances of suicidal behaviour, suicide or deaths in these two trials [5, 6]. In PREVAIL 3 (adjunctive therapy), 6 % of lurasidone and 3 % of placebo recipients withdrew from treatment because of an adverse event [7]. Across the PREVAIL 1and 2 trials, treatment-emergent adverse events occurring at an incidence of C5 % with lurasidone hydrochloride (monotherapy or adjunctive therapy) and at least twice as frequently as with placebo were akathisia, extrapyramidal symptoms (EPS), somnolence, nausea, vomiting, diarrhoea and anxiety [1]. Treatment-emergent adverse events occurring in C2 % of lurasidone hydrochloride recipients and more frequently than in placebo recipients [1]. Lurasidone hydrochloride was associated with small increases in mean serum prolactin levels, which were more pronounced in women than men. Across trials, \1 % of lurasidone hydrochloride recipients had serum prolactin concentrations of C5 times the upper limit of normal [1]. Nevertheless, monitoring of serum prolactin levels may be indicated in some patients
Dosage and Administration
In the USA, lurasidone hydrochloride as monotherapy or as an adjunct to lithium or valproate is approved for use for the treatment of MDEs associated with bipolar I disorder [1]. The recommended dosage in patients with bipolar depression is lurasidone hydrochloride 20–120 mg/day [1]. It is recommended that lurasidone hydrochloride therapy be initiated at a dosage of 20 mg/day; no titration of the initial dosage is required. Lurasidone hydrochloride should be taken with food [1]. The effectiveness of lurasidone hydrochloride for longer-term therapy in bipolar depression beyond 6 weeks, in bipolar II disorder, or in the treatment of mania associated with bipolar disorder has not been established. The ef?cacy and safety of luras
Related articles And Qustion
Lastest Price from Lurasidone hydrochloride manufacturers

US $10.00/KG2025-04-21
- CAS:
- 367514-88-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $2.00/kg2025-04-21
- CAS:
- 367514-88-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 200KG/M