The introduction of Ketone Ester
General description
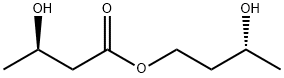
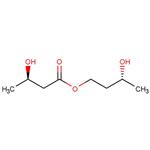
The Ketone Ester, with the CAS No: 1208313-97-6, is also known as (3R)-3-Hydroxybutanoic acid (3R)-3-hydroxybutyl ester. This chemical’s molecular formula is C8H16O4 and molecular weight is 176.21. Its boiling point is 269 ℃ at 760 mm Hg, and its flash point is 101℃. The Ketone Ester is a kind of synthetic intermediate. Its structure is as follows:
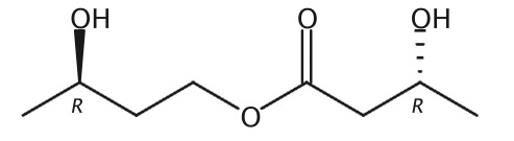
Figure 1 Structure of Ketone Ester.
Chemical synthesis of Ketone Ester
Ketone Ester can be synthesized by 2 steps according to the previous work [1]. (R) - 1,3-butanediol (100.0 g, 1.1 mmol) and triethylamine (224.6 g, 2.22 mol) were diluted in dichloromethane (500 mL). Lower the temperature to 0-5 ° C, add dichloromethane solution of p-toluenesulfonyl chloride (211.6 g, 1.1 mmol) dropwise. After dripping, keep the reaction at 0-5 ° C for 14-18 hours. TLC monitoring response completed. The reaction solution is quenched with water (200mL), stirred at 20-25 ° C for half an hour, and extracted. Add 10% sodium bicarbonate aqueous solution into the organic phase and separate the liquid. The organic phase is dried with anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure to dry (R) - 3-hydroxybutyl-4-methoxybenzenesulfonate (231 g, yield 85%, purity 90%). (R) - 3-hydroxybutyl-4-methoxybenzenesulfonate (100 g, 0.41 mol) was diluted in toluene (1 L). Add R-3-hydroxybutyrate sodium (51.6 g, 0.41 mol) at room temperature. Heat up to 80-90 ℃ and react for 20-24 h. TLC detection response completed. The reaction solution was cooled to room temperature and filtered. The filtrate is decompressed and concentrated to dry. Add methyl-tert-butyl ether (500 mL) into the residue, mix it well, and then filter it. The filtrate is concentrated under reduced pressure to dry (R) - 3-hydroxybutyryl - (R) - 3-hydroxybutyl ester (Ketone Ester) (65 g, yield 90%, purity 95%). 1H NMR (400 MHz, CDCl3) δ 4.39-4.30 (m, 1H), 4.24-4.18 (m, 2H), 3.84-3.93 (m, 1H), 2.87 (s, 2H), 2.49-2.43 (m, 2H), 1.81-1.72 (m, 2H), 1.24 (d, J = 1.7 Hz, 3H), 1.22 (d, J = 1.7 Hz, 3H).
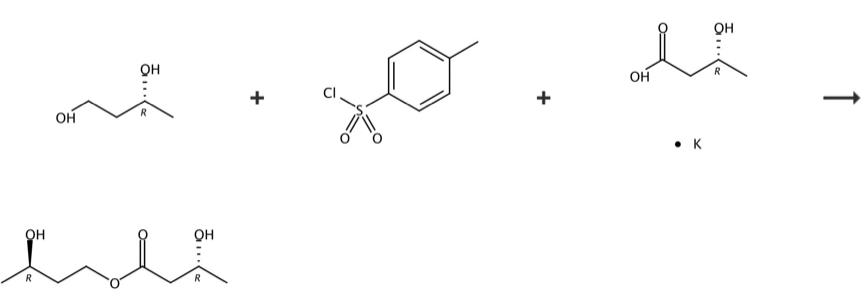
Figure 2 Chemical synthesis of Ketone Ester.
Application of Ketone Ester
Ketone Ester has been developed as an oral source of ketones, which may be utilized for energy, shown to elevate blood ketone levels in humans, as it is fully hydrolyzed to D-β- hydroxybutyrate and (R)-1,3-butanediol following consumption, with the latter being further metabolized to D-β-hydroxybutyrate and acetoacetate in the liver [2]. Ketone monoester also can be delivered in a food form for energy purposes, thereby reducing the potential concerns associated with the administration of a ketogenic diet. In preliminary studies, pair-feeding a diet containing ketone monoester (13.7 g/kg bw/day) to Wistar rats for 66 days did not significantly impact body weights in comparison to a Western diet or a CHO-based diet. Plasma free fatty acid levels and lactate dehydrogenase (LDH) activity were also unaffected [3].
Toxicity of Ketone Ester
In a 28-day toxicity study, Crl:WI (Wistar) rats received diets containing, as 30% of the calories, ketone monoester (12 and 15 g/kg body weight/day for male and female rats, respectively). Control groups received either carbohydrate- or fat-based diets. Rats in the test group consumed less feed and gained less weight than control animals; similar findings have been documented in studies of ketogenic diets. Between-group differences were noted in selected hematology, coagulation, and serum chemistry parameters; however, values were within normal physiological ranges and/or were not accompanied by other changes indicative of toxicity. Upon gross and microscopic evaluation, there were no findings associated with the ketone monoester. In a developmental toxicity study, pregnant Crl:WI (Han) rats were administered 2 g/kg body weight/day ketone monoester or water (control) via gavage on days 6 through 20 of gestation. No Caesarean-sectioning or litter parameters were affected by the test article. The overall incidence of fetal alterations was higher in the test group; however, there were no specific alterations attributable to the test substance [4].
References
[1]Zhang et al. Preparation method of (R)-3-hydroxybutyl (R)-3-hydroxybutanoate from (R)-butane-1,3-diol and (R)-3-hydroxybutyric acid. CN113045416, 29 Jun 2021.
[2]Desrochers S, et al. Metabolism of R- and S-1,3-butanediol in perfused livers from meal-fed and starved rats. Biochem. J. 1992; 285:647–653.
[3]Tate RL, Mehlman MA, Tobin RB. Metabolic fate of 1,3-butanediol in the rat: conversion to - hydroxybutyrate. J. Nutr. 1971; 101:1719–1726.
[4]Clarke K, et al. Oral 28-day and developmental toxicity studies of (R)-3- hydroxybutyl (R)-3-hydroxybutyrate. Regul Toxicol Pharmacol. 2012, 63(2):doi:10.1016/j.yrtph.2012.04.001.
Related articles And Qustion
See also
Lastest Price from Ketone Ester manufacturers

US $0.00/kg2025-08-26
- CAS:
- 1208313-97-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton
US $0.00-0.00/KG2025-05-12
- CAS:
- 1208313-97-6
- Min. Order:
- 1KG
- Purity:
- 97.5%
- Supply Ability:
- 20000