Tetrakis(triphenylphosphine)palladium: Application, toxicity, Preparation
Indication
Tetrakis(triphenylphosphine)palladium[1], also known as Pd(PPh3)4, is a coordination compound with the formula Pd(PPh3)4. It is a yellow-colored crystalline solid that is commonly used as a catalyst in organic synthesis. In particular, it is used in cross-coupling reactions such as the Suzuki coupling reaction and the Heck reaction. The compound is air-stable and soluble in organic solvents such as benzene and chloroform.

Figure 1 Appearance of Tetrakis(triphenylphosphine)palladium
Application
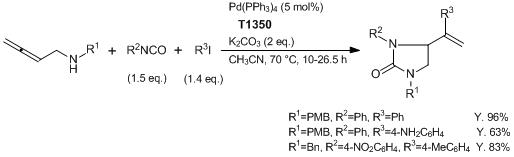
Tetrakis(triphenylphosphine)palladium, commonly known as Pd(PPh3)4, is a popular palladium complex that finds wide application in various organic synthesis reactions. It is a widely used compound in organic chemistry due to its ability to catalyze a variety of reactions. One major application of this compound is in cross-coupling reactions, which involve the formation of a carbon-carbon bond between two different molecules. Specifically, Pd(PPh3)4 is often used in the Suzuki-Miyaura cross-coupling reaction, which can be used to synthesize a wide range of biaryl compounds. In addition to its role in cross-coupling reactions, Pd(PPh3)4 is also used as a catalyst for other organic transformations, including carbon-heteroatom bond formation and olefin hydrogenation. Overall, the versatility of this compound has made it an important tool for synthetic chemists in the development of new organic molecules.
Recycle
Tetrakis(triphenylphosphine)palladium, or Pd(PPh3)4, is a valuable catalyst in organic synthesis, but it can be expensive. Therefore, recycling and reusing this catalyst can be economically beneficial. One common method for recycling Pd(PPh3)4 involves the use of ion-exchange resins. The catalyst is adsorbed onto the resin and then eluted with a suitable solvent to recover the catalyst. The resin can then be regenerated by treatment with an appropriate agent such as hydrochloric acid. This method has been shown to be effective for several types of Pd-catalyzed reactions. Another method for recycling Pd(PPh3)4 involves the use of nanomaterials. For example, palladium nanoparticles supported on carbon nanotubes have been used as an efficient and recyclable catalyst for various organic reactions. After the reaction, the catalyst can be easily recovered by filtration and reused after washing. In addition, Pd(PPh3)4 can also be immobilized on solid supports like silica, alumina, or zeolites, making it easier to separate the catalyst from the reaction mixture. This immobilization process allows the recovery of the catalyst by filtration and subsequent reuse. Overall, several methods are available for the recycling of Pd(PPh3)4, including the use of ion-exchange resins, nanomaterials, and immobilization on solid supports. These methods reduce the cost of the catalyst and make organic synthesis more sustainable[3].
Toxicity
Tetrakis(triphenylphosphine)palladium, also known as Pd(PPh3)4, is a commonly used palladium complex in organic synthesis. While it is effective in catalyzing many reactions, it is important to handle this compound with care due to its potential toxicity.
Inhalation of dust or vapors from Pd(PPh3)4 can lead to irritation of the respiratory tract, including coughing and shortness of breath. Skin contact can cause irritation and redness, and prolonged exposure may lead to skin sensitization. Ingestion of the compound can cause gastrointestinal distress, such as nausea and vomiting.
Studies have also shown that Pd(PPh3)4 has the potential to be carcinogenic and mutagenic, meaning it may increase the risk of cancer and genetic mutations. It is therefore recommended to handle this compound only in a well-ventilated area, with appropriate safety equipment such as gloves and protective eyewear.
It is important to note that Pd(PPh3)4 should only be handled by trained professionals who are familiar with safe laboratory practices and procedures for working with hazardous chemicals. Proper storage, handling, and disposal methods should be followed to minimize the risk of exposure and ensure safety[4].
Storage
Proper storage of Tetrakis(triphenylphosphine)palladium, or Pd(PPh3)4, is essential to maintain its stability and prevent degradation. This compound should be stored in a cool, dry place away from heat sources, oxidizing agents, and other reactive chemicals[5].
It is recommended to store Pd(PPh3)4 in an airtight container, such as a glass ampoule or vial, to minimize exposure to air and moisture. The container should be clearly labeled with the name of the compound, the date of preparation, and any relevant hazard information.
When handling Pd(PPh3)4, it is important to use proper protective equipment to avoid contact with skin, eyes, and respiratory system. Gloves and protective eyewear should be worn at all times when working with this compound.
In addition, it is important to follow proper disposal procedures for Pd(PPh3)4 and any contaminated materials. Unused portions should be stored in a designated hazardous waste container and disposed of according to local regulations.
Overall, proper storage and handling of Pd(PPh3)4 is crucial for maintaining safety in the laboratory and minimizing the risk of exposure and degradation of the compound.
References
[1] Linlin Liu and Bing Yang and Houyu Zhang. Role of Tetrakis(triphenylphosphine)palladium(0) in the Degradation and Optical Properties of Fluorene-Based Compounds[J]. The journal of physical chemistry, C. Nanomaterials and interfaces, 2008, 112(27) : 10273-10278.
[2] Ioele M, Ortaggi G, Scarsella M, Sleiter G. ChemInform Abstract: Oxidation of Terminal Olefins by Hydrogen Peroxide Catalyzed by Tetrakis(triphenylphosphine)palladium(0) [J]. ChemInform,2010,24(28).
[3] He Yulan. Exploration on the method of recovering palladium from tetraphenylphosphine palladium production waste liquid [D]. Zhejiang University of Technology, 2017.
[4] Sahu N, Pal B C. Environmental and health hazards of Tetrakis(triphenylphosphine)palladium. Journal of Environmental Science and Health, Part C,2017, 35(1), 1-18.
[5] Zhang X, Liu B, Chen Y. Toxicity and environmental impact of Tetrakis(triphenylphosphine)palladium: A review. Chemosphere, 2015,120, 552-558.
Related articles And Qustion
See also
Lastest Price from Tetrakis(triphenylphosphine)palladium manufacturers

US $1.00/g2025-06-02
- CAS:
- 14221-01-3
- Min. Order:
- 1g
- Purity:
- 0.99
- Supply Ability:
- 20 tons

US $0.00/kg2025-05-15
- CAS:
- 14221-01-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg