Tetracaine: pharmacodynamic, pharmacokinetics and side effects
General Description
Tetracaine medicated plaster is a local anesthetic patch containing lidocaine and tetracaine. It works by blocking sodium channels, halting nerve impulses. When applied to the skin with controlled heat-assisted delivery, the patch raises skin temperature, enhancing tetracaine diffusion. Clinical trials showed superior anesthesia compared to placebo, with minimal impact on vital signs. Tetracaine has bactericidal and antiviral properties at higher concentrations. Systemic absorption depends on the number of plasters, duration of application, and skin characteristics. Adverse events are generally mild and include erythema, edema, pruritus, and burning sensation. Follow dosage guidelines, avoid use in certain conditions, compromised skin, and contact with eyes.

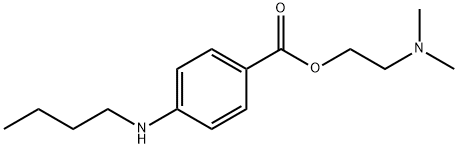
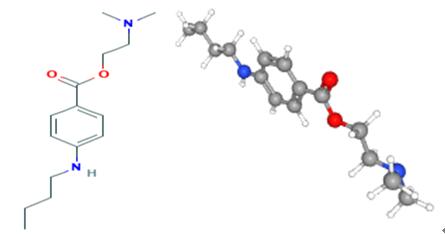
Figure 1. Tetracaine
Pharmacodynamic
Tetracaine are local anesthetics that work by blocking voltage-gated sodium channels to halt the generation and conduction of nerve impulses. The tetracaine medicated plaster consists of a patch containing 70 mg/70 mg of lidocaine and tetracaine, along with a controlled heat-assisted drug delivery pod. When the plaster is applied to the skin and exposed to oxygen, an exothermic reaction occurs in the heating pod, raising the patch's temperature to around 26-34°C and increasing the adjacent skin temperature by approximately 5°C. This temperature elevation enhances the diffusion of tetracaine into the dermis. In a clinical trial with healthy volunteers, the tetracaine medicated plaster demonstrated superior surface anesthesia compared to a placebo. After a 30-minute application, the anesthetic depth and duration were significantly greater with the tetracaine plaster. Vital signs such as blood pressure and pulse rates were unaffected by the plaster over a 2-hour evaluation period. Additionally, tetracaine exhibits bactericidal and antiviral activity at concentrations above 0.5-2%. However, the impact on the effectiveness of live vaccines administered after using the plaster is unknown, emphasizing the need for close monitoring of immune responses. 1
Pharmacokinetics
Tetracaine, when administered through the tetracaine medicated plaster, has pharmacokinetic properties influenced by various factors. The systemic exposure to tetracaine depends on the number of plasters applied, duration of application, and characteristics of the skin. The heating pod component of the plaster does not appear to affect the systemic absorption or hasten the process. In adult patients, applying two plasters for 60 minutes resulted in a peak plasma concentration of tetracaine below 9 ng/mL, while tetracaine concentrations were undetectable. Different age groups showed varying lidocaine Cmax levels: 9 ng/mL in elderly patients (65 years), 6 ng/mL in children aged 3-6 years, and 2.1 ng/mL in children aged 7-12 years. Tetracaine concentrations remained below 0.9 ng/mL for all age groups. Tetracaine undergoes rapid metabolism in the skin and plasma by unspecified esterases. Due to rapid hydrolysis by esterases, the clearance rate and half-life of tetracaine in humans have not been determined. As for drug interactions, caution is advised when coadministering the tetracaine medicated plaster with certain antiarrhythmic drugs, as it may result in additional systemic toxicity. However, no pharmacokinetic data exist for the plaster in patients with cardiac, renal, or hepatic impairment, although tetracaine's half-life might be prolonged in patients with cardiac or hepatic dysfunction. 2
Side effect
Tetracaine is generally well tolerated when used in the lidocaine/tetracaine medicated plaster for minor dermatological procedures and vascular access procedures. Treatment-related adverse events were mild to moderate and primarily included erythema (2-30%), oedema (11%), pruritus (5%), and burning sensation (2%). These adverse events typically resolved on their own after treatment. The incidence and nature of adverse events associated with the lidocaine/tetracaine plaster were similar to those observed in placebo or unheated plaster treatment arms. However, compared to lidocaine/prilocaine cream or plaster, recipients of the lidocaine/tetracaine plaster had a significantly higher incidence of erythema. Common adverse events among recipients of the lidocaine/tetracaine plaster included erythema (71%), blanching (12%), and oedema (12%). Less common adverse events (1%) may include rash, contact dermatitis, application site reaction, urticaria, skin discoloration, pain, and taste perversion. It is important to follow the recommended dosage and administration guidelines for the lidocaine/tetracaine plaster and consult local prescribing information for contraindications, warnings, and recommendations. Use with caution in patients with severe hepatic, renal, or cardiac impairment, avoid use on mucous membranes or compromised skin barriers, and avoid contact with the eyes. 3
Reference
1. Shomaker TS, Zhang J, Love G, et al. Evaluating skin anesthesia after administration of a local anesthetic system consisting of an S-Caine patch and a controlled heat-aided drug delivery (CHADD) patch in volunteers. Clin J Pain, 2000, 16(3): 200-204
2. Eurocept International. Rapydan 70 mg/70 mg medicated plaster: summary of product characteristics. 2010.
3. Croxtall JD. Lidocaine/tetracaine medicated plaster: in minor dermatological and needle puncture procedures. Drugs, 2010, 70(16):2113-2120.
You may like
Related articles And Qustion
See also
Lastest Price from Tetracaine manufacturers

US $0.00-0.00/kg2025-07-12
- CAS:
- 94-24-6
- Min. Order:
- 1kg
- Purity:
- 99%pure
- Supply Ability:
- 1000kg

US $1.00/kg2025-06-10
- CAS:
- 94-24-6
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 2000kgs