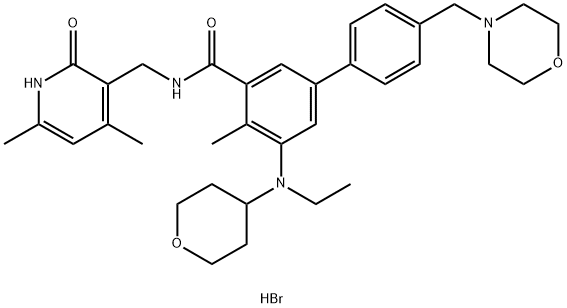
Tazemetostat Hydrobromide:Synthesis and Introduction
Synthesis of Tazemetostat Hydrobromide
Tazemetostat Hydrobromide is synthesised using 2- methyl-3-nitrobenzoic acid as a raw material by chemical reaction. The specific synthesis steps are as follows:
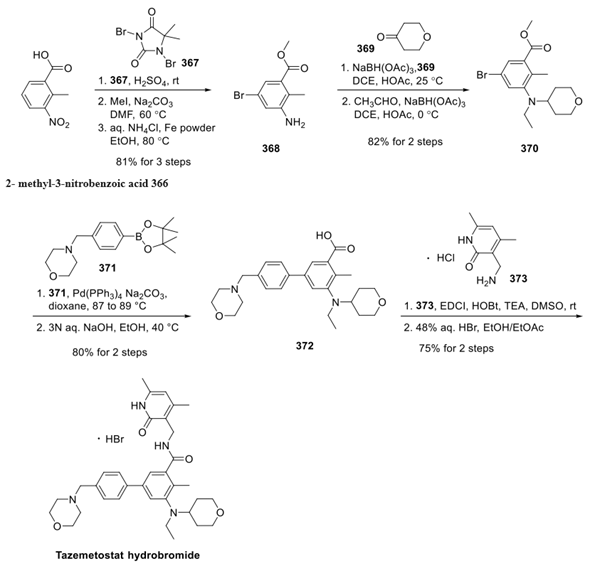
The synthesis began with the bromination of 2- methyl-3-nitrobenzoic acid 366 by dibromantin 367 in sulfuric acid. Esterifcation and nitro reduction generated aniline 368 in 81% overall yield. Arene 368 was then converted to dialkylarylamine 370 by a sequence of two reductive aminations, first with 4-oxanone (369) and second with acetaldehyde, furnishing 370 in 82% yield from 368. Suzuki coupling with boronate 371 followed by saponification provided compound 372 in 80% yield over 2 steps. Finally, amide bond formation between the carboxylic acid of 372 and amine 373 was accomplished using standard conditions, and the resulting product was treated with aqueous hydrobromic acid to provide tazemetostat hydrobromide in 75% yield over two steps.
Introduction of Tazemetostat Hydrobromide
Tazemetostat hydrobromide is an enhancer of the zeste homologue 2 (EZH2) inhibitor developed by Epizyme in collaboration with Eisai and approved by the USFDA for the treatment of relapsed or refractory follicular lymphoma. EZH2 is a histone methyl transferase that plays an essential role in B-lymphocyte function and proliferation. Overexpression or mutation of EZH2 has been observed in many lymphomas, and inhibition of EZH2 by tazemetostat elicits an antitumor effect. Tazemetostat is dosed as an 800 mg tablet, twice daily.