How is Tepotinib Synthesised?
Synthesis of Tepotinib
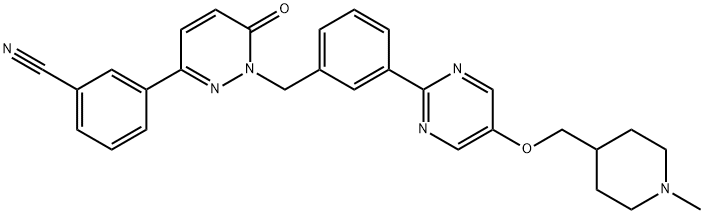
Tepotinib is synthesised through a two-step chemical reaction using acetylbenzonitrile as the raw material. The specific synthesis steps are as follows:
Step 1: Preparation of Tepotinib Nitrile
Glyoxylic acid 375 is introduced to acetylbenzonitrile 374 in acetic acid and later exposed to hydrazine hydrate.327,328 A separate treatment with acetic acid yielded arene 376 as a crystalline solid in 43% yield in 3 steps. Separately, a selective Suzuki cross-coupling of aryl boronate 377 and aryl iodide 378 delivered biaryl intermediate 379 in 72% yield. The alcohol of 379 was then converted to the corresponding alkyl chloride, which in turn was reacted with 376 in the presence of potassium carbonate and NMP to afford 380 in excellent yield.
Step 2: Preparation of Tepotinib
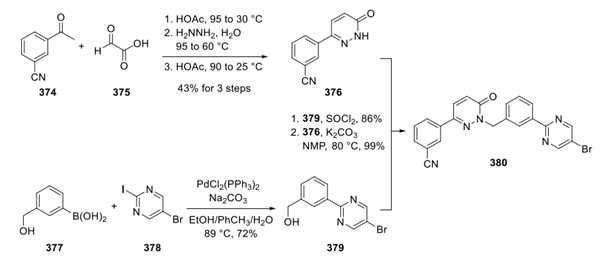
The bromide of 380 was then converted into the corresponding pinacol broronate 381 under standard conditions and isolated in 77% yield. Conversion of the pinacol boronate 381 to the corresponding phenol 382 in excellent yield was accomplished using sodium perborate tetrahydrate. Lastly, phenol 382 underwent an efficient Mitsunobu reaction with 383 to give the corresponding piperidine intermediate, which then was subjected to acidic conditions with formic acid to effect removal of the Boc group. Methylation of the piperidine ring was accomplished using aqueous formaldehyde under Eschweiler-Clarke conditions, which resulted in tepotinib as a crystalline solid in 71% yield after recrystallization from isopropyl alcohol.
You may like
See also
Lastest Price from Tepotinib manufacturers
US $0.00-0.00/mg2025-04-18
- CAS:
- 1100598-32-0
- Min. Order:
- 10mg
- Purity:
- 95%+
- Supply Ability:
- 100000

US $0.00-0.00/kg2024-11-26
- CAS:
- 1100598-32-0
- Min. Order:
- 1kg
- Purity:
- 99%, Single impurity<0.1
- Supply Ability:
- 1 ton