Talc: mineral, Uses and Safety
Talc mineral
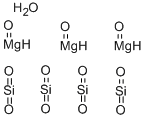
Talc is a naturally occurring monocrystalline or tricrystalline system of silicate pure minerals, white, brown, grey and green in appearance, mined from rocky sediments in the earth's crust, and consisting of hydrogen, magnesium, oxygen and silicon. Talc forms mica-like flakes. Talc is the softest mineral on the Mohs hardness scale of 1 and can be easily cut and crushed. Talc has perfect resolution in one direction. This means that it breaks up into thin flakes.

Asbestos is also a naturally occurring silicate mineral with a different crystal structure. Unlike talc, asbestos is a known carcinogen when inhaled. Because talc and asbestos are minerals in close proximity, when talc was mined it could contain traces of asbestos, creating the problem of possible asbestos contamination of talc.
Uses of Talc
Talc is a naturally hydrophobic gangue mineral in most sulfide ores. Talc is highly chemically stable, soft, lubricating, heat-resistant and absorbent, resulting in a wide range of applications in the cosmetic (common ingredient in powders, setting powders, eye shadows, blushes, foundations and creams), paper, paint and pharmaceutical industries. It can be used as an ingredient in ceramics, paper, paints, roofing, plastics, cosmetics and baby talcum powder, in the manufacture of rubber and plastics; to reduce friction and wear and tear in the automotive industry; to dilute coloured products in cosmetics and act as a filler; and talc pleural fixation for the treatment of patients with malignant pleural effusion (MPE).
Safety of Talc
Pure talc is usually safe, but talc contaminated with asbestos poses serious health risks. The talc industry and the U.S. Food and Drug Administration (FDA) have claimed that talc has been asbestos-free since 1976, when the talc industry established a voluntary specification for the asbestos content of cosmetic talc. However, recent evidence shows that cosmetic talc is not asbestos-free and never has been. Under the Federal Food, Drug, and Cosmetic Act (FD&C Act), cosmetic products and ingredients, with the exception of colour additives, are not required to be reviewed or approved by the FDA prior to marketing. Cosmetics must be properly labelled and must be safe for use by the consumer under the conditions of use or customary use indicated on the label.
In addition, the FDA released 2023 data on asbestos testing of cosmetics containing talc. These products were selected based on a variety of factors, including the type of talc-containing cosmetics, price range, popular products in social media and advertising, products marketed to children, products marketed to women of colour, and third-party reports of potential asbestos contamination, if any. Sample testing was conducted by AMA Analytical Services, Inc.(AMA) on behalf of the FDA. Samples were analysed using polarized light microscopy (PLM) and transmission electron microscopy (TEM).
FDA Releases Data from the Agency's 2023 Testing of Talc-Containing Cosmetic Products for Asbestos. https://www.fda.gov/cosmetics/cosmetics-news-events/fda-releases-data-agencys-2023-testing-talc-containing-cosmetic-products-asbestos
References:
[1] ELMASRY A, AMR M, EL-DOMIATY H, et al. Thoracoscopic Talc Insufflation Versus Talc Slurry in Patients with Malignant Pleural Effusion[C]. 2021. DOI:10.21608/SCUMJ.2021.161152.
[2] TESS BIRD. A Review of the Talc Industry's Influence on Federal Regulation and Scientific Standards for Asbestos in Talc.[J]. New Solutions-A Journal of Environmental and Occupational Health Policy, 2021. DOI:10.1177/1048291121996645.
[3] JULY ANN BAZAR. Talc Flotation—An Overview[J]. Minerals, 2021. DOI:10.3390/min11070662.
Related articles And Qustion
See also
Lastest Price from Talc manufacturers

US $0.00-0.00/kg2025-07-25
- CAS:
- 14807-96-6
- Min. Order:
- 1kg
- Purity:
- 98.5%
- Supply Ability:
- 1000kg

US $120.00/kg2025-04-21
- CAS:
- 14807-96-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000000