Synthetic route to the insecticide Dicloromezotiaz
Synthesis of Dicloromezotiaz
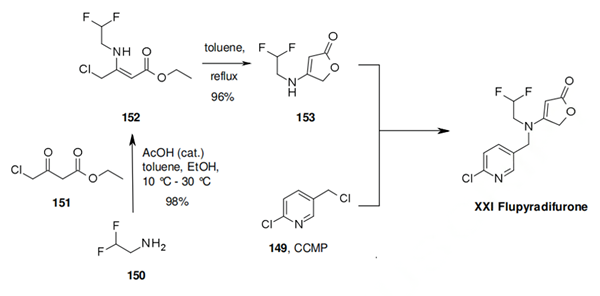
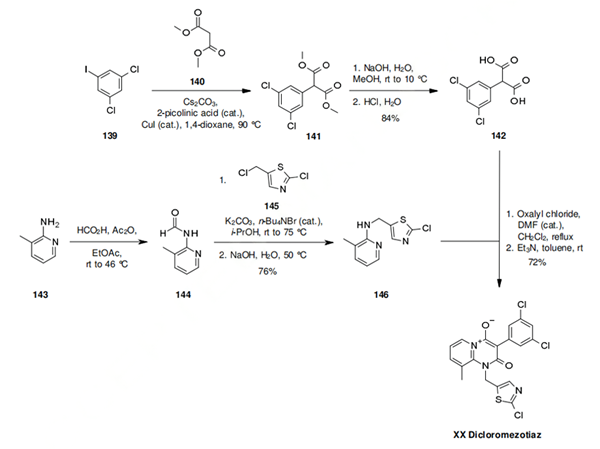
The synthesis of dicloromezotiaz is similar to that of triflumezopyrim. The convergent synthesis begins with a copper catalyzed coupling of dimethyl malonate 140 with the iodide 139. The crude product 141 is directly hydrolysed to the diacid 142 in overall yield of 84% for the two steps. The required coupling partner 146 is prepared efficiently by formamide protection of 143, followed by phase transfer catalyzed alkylation of 144 with 2-chloro-5-(chloromethyl)thiazole (145), followed by in situ deprotection, to yield 146 in 76% yield. To complete the synthesis, the diacid is activated as the diacid chloride and treated with 146 in toluene in the presence of triethyl amine. This gives dicloromezotiaz in 72% yield as a single crystal polymorph.
Introduction of Dicloromezotiaz
Dicloromezotiaz is the second mesoionic insecticide from DuPont, which has the same mode of action as triflumezopyrim. The compound is structurally very similar to triflumezopyrim, having a mesoionic central core, a meta-substituted aromatic (in this case a dichloro benzene) and a methylene heteroaryl substituent on the nitrogen. The only other difference is the additional methyl substituent on the amino pyridyl moiety. Whilst triflumezopyrim is a hopper specialist, dicloromezotiaz has good activity on a range of Lepidopteran species.
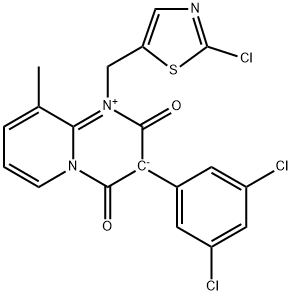 2H-Pyrido[1,2-a]pyrimidinium, 1-[(2-chloro-5-thiazolyl)methyl]-3-(3,5-dichlorophenyl)-3,4-dihydro-9-methyl-2,4-dioxo-, inner salt
2H-Pyrido[1,2-a]pyrimidinium, 1-[(2-chloro-5-thiazolyl)methyl]-3-(3,5-dichlorophenyl)-3,4-dihydro-9-methyl-2,4-dioxo-, inner salt 1263629-39-5