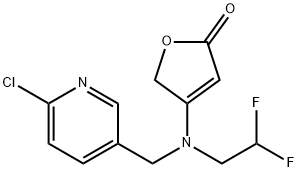
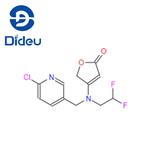
Synthetic route to the insecticide Flupyradifurone
Synthesis of Flupyradifurone
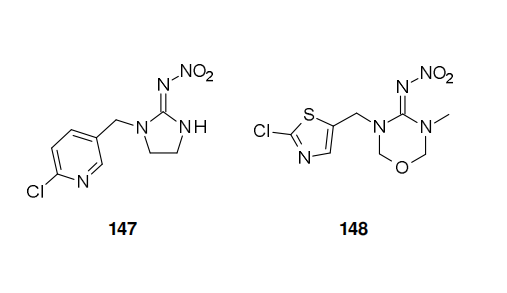
Technical synthesis of flupyradifurone (XXI) might involve 6-chloro-3-chloromethyl-pyridine (149, CCMP) as a key intermediate, as this is also a key intermediate of Bayer CropScience's imidachloprid (147). There are two possible technical syntheses of XXI.
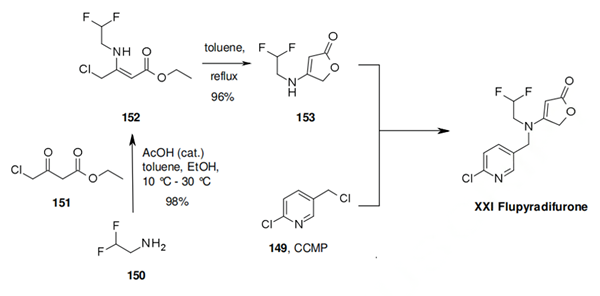
Scheme one: reaction of 2,2-difluoroethanamine (150) with ethyl 4-chloro-3-oxobutanoate (151) under acidic catalysis leads to 152 in excellent yield. Subsequent heating of 152 in toluene leads to the furanone 153. Alkylation of 153 with CCMP (149) would in principle provide flupyradifurone (XXI). This actual alkylation is not described but very similar chemistry has been reported by Bayer CropScience chemists.
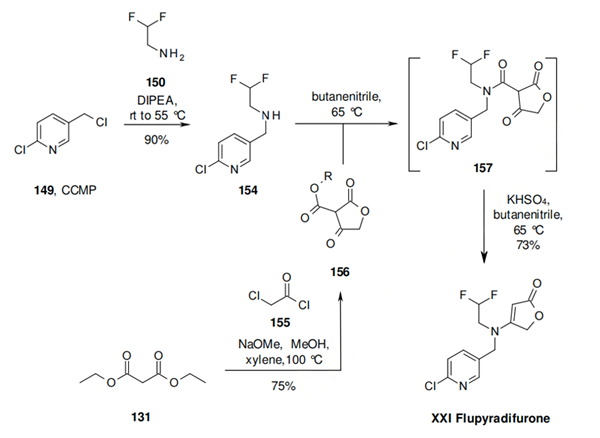
Scheme two: Condensation of diethyl malonate (131) and chloroacetyl chloride (155) under basic conditions leads to tetronic acid derivative 156 as a mixture of methyl and ethyl esters. This is coupled with 154, which was prepared by reaction of CCMP (149) with 2,2-difluoroethanamine (150), to give the amide 157. This can be isolated, but is most likely rearranged to flupyradifurone (XXI) by heating in butyronitrile by a ring opening-ring closing mechanism followed by decarboxylation in good yield. The key intermediate amine 150 required for both of these synthesis can be prepared from 2-chloro-1,1-difluoro-ethane either directly by treatment with ammonia under high pressure conditions, or by alkylation of 2-chloro-1,1-difluoro-ethane with a protected amine, followed by deprotection.
Introduction of Flupyradifurone
Flupyradifurone (XXI, Bayer CropScience), is proposed as an alternative insecticide for controlling sucking pest species. Foliar applications, and soil drenches of the end-use product, SivantoTM 200 SL, are approved for use on a large number of crops, including fruits and vegetables. Flupyradifurone (XXI) acts on insect nicotinic acetylcholine receptors as has been shown in radioligand binding studies conducted with tritiated imidacloprid (147). Whilst imidacloprid (147) and thiamethoxam (148) are nicotinic acetylcholine receptor competive modulators, belonging to the IRAC class group 4A, flupyradifurone (XXI) in contrast belongs to the butenolide sub-group 4D.
You may like
See also
Lastest Price from Flupyradifurone manufacturers
US $0.00-0.00/KG2025-06-03
- CAS:
- 951659-40-8
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS