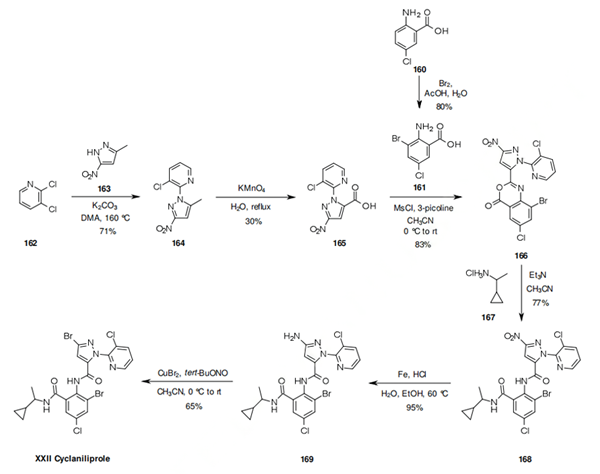
Synthetic route to the insecticide Cyclaniliprole
Synthesis of Cyclaniliprole
One convergent route explored by ISK for the synthesis of cyclaniliprole involves the coupling of anthranilic acid 161 and pyrazole carboxylic acid 165. The anthranilic acid 161 in turn was synthesized by bromination of 160. The first step toward the synthesis of 165 involves reaction of pyrazole 163 and pyridine 162 in the presence of potassium carbonate at 160 °C, which affords the pyrazolylpyridine 164. Oxidation of 164 then yields the key intermediate 165 which is reacted together with 161 in the presence of methanesulfonyl chloride and 3-picoline to yield benzoxazinone 166. Reaction of 166 with amine 167 yields the anthranilic diamide functionality of 168. Béchamp reduction of 168 gives the aminopyrazole 169, the aniline function of which is converted to the bromine by Sandmeyer reaction to give cyclaniliprole (XXII).
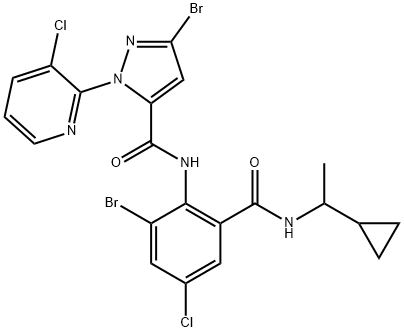
Introduction of Cyclaniliprole
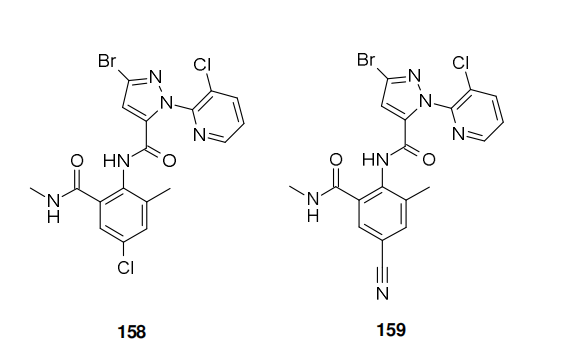
ISK has presented cyclaniliprole (XXII) as a new anthranilic diamide insecticide in 2013. It is related to DuPont's commercial products chlorantraniliprole (158) and cyantraniliprole (159), both structurally and by its mode of action as all three are classified as ryanodine receptor modulators. Each of these active ingredients bears the 5-bromo-2-(3-chloro- 2-pyridyl)pyrazole-3-carboxamid moiety, differing from one another solely by substitution on the anthranillic acid portion of the molecules.