Synthetic methods of methyl levulinate
Introduction
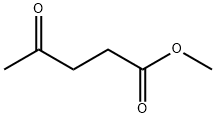
Methyl levulinates (ML;Figure.1) have been identified as high potential applications in the transportation of diesel, additive for gasoline, flavoring and fragrance industries, less toxicity, more lubricity, and steady flash point with excellent fluidity under very low-temperature reaction conditions.[1] As a derivative of levulinic acid (LA), methyl levulinate is neutral and has a lower boiling point than levulinic acid. Methyl levulinate can be used not only as a platform chemical like levulinic acid,but also as a direct additive for conventional fuels toward high performance. A lot of attempts have been made for production of methyl levulinate from cellulose, among which homogeneous acidic catalysts (e.g.,H2SO4, special ionic liquids, etc) or mixtures of Lewis and Brønsted acids(e.g., indium trifluoromethanesulfonate and 2-naphthalenesulfonicacid) were generally employed to achieve high yield of methyl levulinate. However, the recovery or high cost of those catalysts is still the main challenge for large-scale application.[2]

Synthetic methods of methyl levulinate
Production of methyl levulinate from cellulose
Gao and his co-workers herein proposed an earth-abundant cobalt pyrite (CoS2) as a heterogeneous catalyst for the conversion of cellulose to methyl levulinate,which has high potentials in industrial production of methyl levulinate because of its high activity and easy recovery. Since the performances of catalysts obtained from different manufacturers were dramatically different, the structure-activity relationship of the two kinds of CoS2 was particularly examined, indicating the great importance of crystal facet. The highest yield of methyl levulinate reached 61mol% under the tested conditions of 200℃, 2MPa initial pressure, 0.45 catalyst/cellulose mass ratio, and 3h reaction time. The XRD and TEM analysesdemonstrated the crystal facet (111) of cobalt disulfide as a robust active site, which was in good agreement with the highest acidity of the crystal facet (111) calculated by the work functions. The XPS characterization showed that the main chemical valence of cobalt disulfide responsible for the methyl levulinate production was the surface Co2+ species. This study is valuable for the development of a recoverable catalyst for the cellulose to methyl levulinate process.[2]
Efficient Conversion of Levulinic Acid to Methyl Levulinate
The present work focus on the synthesis of x% SO42- loaded SnO₂ nanocatalyst at different percentages (x=2.3, 4.6, 6.9, 9.2, and 11.5% w/w) for acid-catalyzed esterification. The synthesized catalysts were characterized by X-ray powder diffraction (XRD), fourier transform infrared spectroscopy (FT-IR), brunauer-emmett-teller (BET), scanning electron microscopy (SEM), transmission electron microscope (TEM), temperature-programmed desorption, and thermal gravimetric analysis (TGA/DTA) techniques. Methyl levulinate is an important chemical feedstock and its production through a catalytic route has been explored in this work. All the synthesized catalysts show excellent catalytic activity towards liquid-phase esterification of levulinic acid (LA) in atmospheric pressure under non-corrosive and mild conditions. The various reaction parameters like the effect of sulfate content, reaction temperature, reaction time, molar ratio of reactants, and prepared catalyst weights are optimized to get the highest conversion and selectivity of methyl levulinates. Among them, as prepared 6.9% SO42- /SnO₂ catalyst exhibits the highest conversation of levulinic acid (98%) and more selectivity towards methyl levulinate (100%). The catalytic stability and recyclability of the most active nanocatalysts were performed up to five successive runs and proves less variation in the catalytic activity.[1]
Conversion Poplar Wood toward Methyl Levulinate
In this study, to further valorize the hemicellulose, Zhai et al. exploreda one-step transformation of C5 sugar (C5 glycoside) derived from biomass into methyl levulinate in a dimethoxymethane/methanol cosolvent with an acid catalyst. Here, methanolysis treatment was used to release the hemicellulose sugar (methyl glycoside) and lignin fragments from poplar sawdust, leaving behind a cellulose-rich solid substrate (cellulose pulp). Various acid catalysts and strategy achieved the fractionation and sequential utilization of all the biomass components. The distribution of methyl glycosides and delignification was dependent on the presence of acid catalysts and reaction temperatures. The obtained lignin fraction was separated into solid lignin fragments and liquid lignin oil according to their molecular weight distribution. Subsequently, directional conversion of methyl C5 glycosides into methyl levulinate was performed with dimethoxymethane/methanol as the cosolvent. A yield of 12−30% of methyl levulinate yield (based on the methyl glycoside) was achieved under these conditions. The remaining cellulose rich substrate showed enhanced susceptibility to enzymatic hydrolysis, resulting in a yield of glucose of above 70%. Overall, the described strategy shows practical implications for the effective valorization of biomass.[3]
Catalytic conversion of duckweed to methyl levulinate
Duckweed (Lemna minor) is a fast-growing aquatic plant, which can be found in many warm and humid regions. As an energy plant, it is abundant, widely distributed, easily harvested and uncompetitive with most grains and vegetables for arable land. What's more important is that this nonedible renewable resource contains low lignin but high starch content (it normally is higher than 30 wt%. This unique composition of duckweed makes it a good alternative for levulinic acid production in industrial biorefinery since many previous studies showed that starch is more flexible for saccharification than cellulose. Therefore, in this study, an effective strategy for duckweed conversion to LA and its methyl ester is proposed over the environmental friendly catalyst of acidic ionic liquids (ILs). The results show that IL structure has a significant effect on its acidic strength, which finally determines the process efficiency for levulinate methyl generation. With the optimized catalyst of [C3H6SO3HPy]HSO4, 88.0% duckweed is consumed, resulting in a comparable methyl levulinate yield of 73.7% and a process efficiency of 81.8% at 170°C for 5h. Furthermore, this process is substantially influenced by the reaction condition, particularly, it is significantly temperature-dependent. In addition, solvent has a remarkable intensified effect on the process efficiency, which dramatically decreases from 81.8 to 53.7% when methanol is replaced by water.[4]
References
1. Mathivanan D, Mani D, Saranraj K, Archana S, Sengottaiyan C, Chang H. Efficient Conversion of Levulinic Acid to Methyl Levulinate Over SO2-?/SnO? Nanocatalysts. J Nanosci Nanotechnol. 2021;21(6):3237-3248. doi:10.1166/jnn.2021.19323
2.Gao W, Wu G, Zhu X, et al. Production of methyl levulinate from cellulose over cobalt disulfide: The importance of the crystal facet (111). Bioresour Technol. 2022;347:126436. doi:10.1016/j.biortech.2021.126436
3.Zhai Q, Hse CY, Long F, et al. Methanolysis Fractionation and Catalytic Conversion of Poplar Wood toward Methyl Levulinate, Phenolics, and Glucose. J Agric Food Chem. 2019;67(35):9840-9850. doi:10.1021/acs.jafc.9b03806
4.Chen Z, Ma X, Xu L, Wang Y, Long J. Catalytic conversion of duckweed to methyl levulinate in the presence of acidic ionic liquids. Bioresour Technol. 2018;268:488-495. doi:10.1016/j.biortech.2018.08.033
See also
Lastest Price from Methyl levulinate manufacturers
US $10.00/KG2025-04-21
- CAS:
- 624-45-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $10.00/kg2025-04-21
- CAS:
- 624-45-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 ton