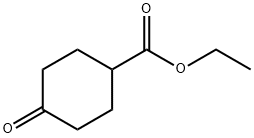
Synthetic applications of Ethyl 4-oxocyclohexanecarboxylate
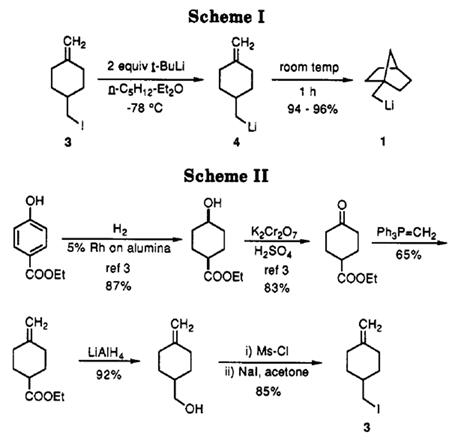
Ethyl 4-oxocyclohexanecarboxylate(4-cyclohexanonecarboxylic acid ethyl ester; ethyl 4-oxocyclohexancarboxylate; ethyl 4-cyclohexanonecarboxylate; C9H14O3)is a chemical substance which has many synthesis routes and also can be used as raw material for many other downstream products[2].Such as used in the synthesis of (1-norbornylmethy1) lithium. According to William F. Bailey’s study, a highly efficient, alternative route to (1-norbornylmethyl) lithium that provided 1 in yields of 94-96% was reported which had two-step, one-pot synthetic sequence, involving 5-exo cyclization of the 5-hexenyllithium derived from 4-(iodomethyl)-l-methylenecyclohexane, is summarized in Scheme I. The iodide precursor, 3, is prepared from ethyl 4-hydroxybenzoate, via commercially available ethyl 4-oxocyclohexanecarboxylate, as depicted in SchemeⅡ[3]:
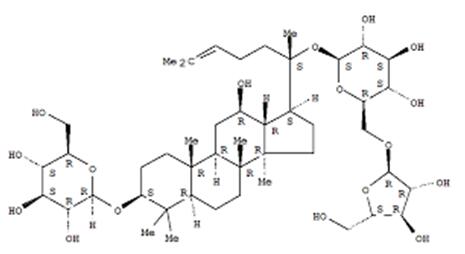
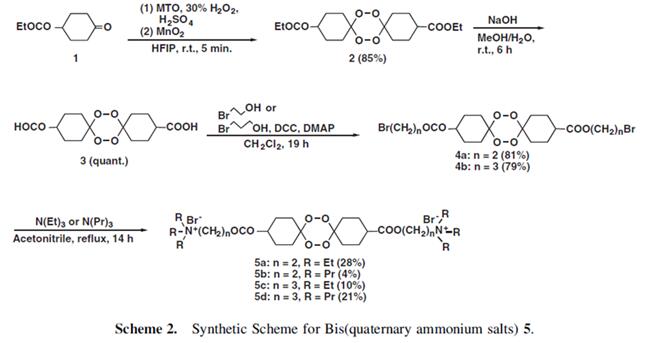
Ethyl 4-oxocyclohexanecarboxylate also can be used to prepare novel water-soluble conjugates of 1,2,4,5-tetraoxane bis(quaternary ammonium salts) which were synthesized in a relatively stable crystalline form via four steps starting from methyltrioxorhenium-catalyzed endo-peroxidation of ethyl 4-oxocyclohexanecarboxylate with hydrogen peroxide in hexafluoro-2-propanol. And the in vitro toxicity of water-soluble tetraoxanes 5a-5d which synthesized by above method to malaria parasites indicated that they were inactive against the Plasmodium falciparum FCR-3 strain[5]:
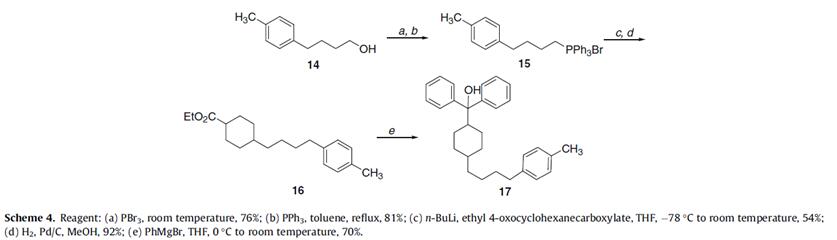
Besides, ethyl 4-oxocyclohexanecarboxylate can be used in the preparation process of terfenadine analogs which can contribute to hERG binding and antihistamine activity, in order to determine the contribution of the tertiary amine of the central piperidine ring to hERG binding and H1 activity, the cyclohexyl analog 17 was prepared (Scheme 4) which used ethyl 4-oxocyclohexanecarboxylate[6]:
Reference
[1]https://pubchem.ncbi.nlm.nih.gov/compound/Ethyl-4-oxocyclohexanecarboxylate
[2]http://www.molbase.com/en/synthesis_17159-79-4-moldata-9717.html#tabs
[3]William F. Bailey, Atmaram D. Khanolkar. Preparation of (1-Norbornylmethy1) lithium[J]. Organometallics, 1993, 12: 239-240.
[4]Shuzhong Wang, Kazuhiko Hayashi, Shinsuke Kaga, Kazuya Oharu. Process for producing a fluorine-containing compound[P]. 2004, US20040024269A1.
[5]Naokazu Kumura, Hirotaka Furukawa, et al. Synthesis of Novel Conjugates of Tetraoxane Endoperoxide with Bis(Quaternary Ammonium Salts)[J]. Biosci. Biotechnol. Biochem, 2009, 73(1): 217-220.
[6]Robert Aslanian , John J. Piwinski, Xiaohong Zhu, Tony Priestley, Steve Sorota, Xiao-Yi Du, Xue-Song Zhang, Robbie L. McLeod, Robert E. West, Shirley M. Williams, John A. Hey. Structural determinants for histamine H1 affinity, hERG affinity and QTc prolongation in a series of terfenadine analogs[J]. Bioorganic & Medicinal Chemistry Letters, 2009, 19: 5043-5047.
Related articles And Qustion
See also
Lastest Price from Ethyl 4-oxocyclohexanecarboxylate manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 17159-79-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt

US $20.00/KG2025-04-21
- CAS:
- 17159-79-4
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 50MT/year