Synthesis, Properties, Chemical Reactivity, Reactions of 1,2-Dithiolane
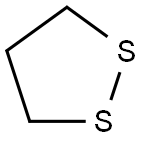
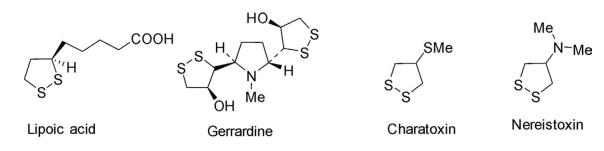
1,2-Dithiolane is a fully saturated, five-membered, sulfur heterocycle with two vicinal sulfur atoms and three carbon atoms in the ring. This class of compounds can be regarded as cyclic dithioacetals or ketals and are widely distributed in nature. They are isolated from animal tissues ranging from eukaryotes to mammals and also from lower to higher plants.
Physical Properties
1 H NMR (CD3 CN), δ (ppm): C3 –H, 3.36; C4 –H, 2.09; C5 –H, 3.36.
13C NMR (TFA-d), δ (ppm): C3 , 41; C4 , 56; C5 , 41.
Some of the important natural products are illustrated in the following diagram.
Synthesis
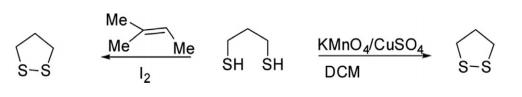
The parent 1,2-dithiolane has been obtained by oxidative cyclization of propane-1,3-dithiol with iodine in the presence of 2-methylbut-2-ene or with potassium permanganate adsorbed on copper sulfate.
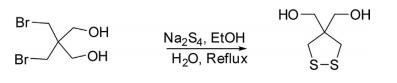
1,2-Dithiolane-4,4-diyldimethanol has been prepared by the reaction of sodium tetrasulfide with 2,2-bis(bromomethyl)propane-1,3-diol in good yields.
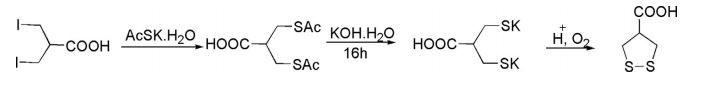
3-Iodo-2-(iodomethyl) propionic acid on treatment with potassium salt of thioacetic acid and oxygen afforded 1,2-dithiolane-4-carboxylic acid.
Chemical Reactivity
Sulfur in 1,2-dithiolane is nucleophilic in nature and easily alkylated with alkyl halide or trimethyloxonium tetrafluoroborate to form 1,2-dithiolium salts.
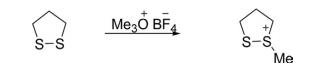
Ring-Opening and -Expansion Reactions
Nucleophiles derived from alkyl lithium, Grignard reagents, and cyanide ion directly attack sulfur atoms to form ring-opened products. Lithium acetylide on reaction with 1,2-dithiolane afforded ring-enlarged products.
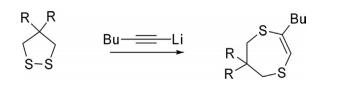
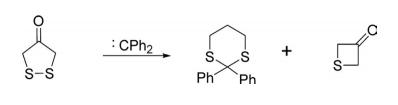
Reactions With Carbenes
1,2-Dithiolanes on reaction with diphenylcarbenes afforded a mixture of inserted and desulfurized products.
Reduction
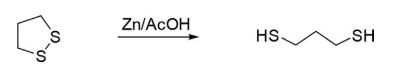
1,2-Dithiolane on reduction with Zn/HCl gave propane-1,3-dithiol.