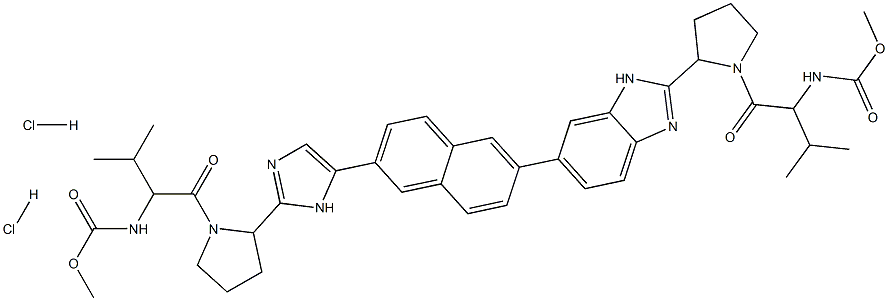
Synthesis of Ravidasvir Hydrochloride
Synthesis of Ravidasvir Hydrochloride
Ravidasvir Hydrochloride is synthesised using 2-Bromonaphthalene as a raw material by chemical reaction. The specific synthesis method is as follows:

The naphthalene−imidazole portion of the core was assembled in three steps from 2-bromonaphthalene. Accordingly, 40 underwent Friedel−Crafts acylation with chloroacetyl chloride in the presence of aluminum chloride. The resultant α- chloroketone was alkylated with N-Boc-L-proline (41) to give a diketo intermediate, which cyclized to imidazole 42 upon heating in the presence of ammonium acetate. Miyaura borylation of bromide 42 provided the corresponding boronic ester, which was coupled with benzimidazole 43 under Suzuki conditionsto convergently assemble the benzimidazole−naphthalene−imidazole core 44. Acidic removal of the Boc groups followed by bis-acylation with N-Moc-L-valine (45) using EDCI generated ravidasvir freebase. Ravidasvir hydrochloride was prepared by treatment with HCl followed by crystallization from ethanol and n-butyl acetate.
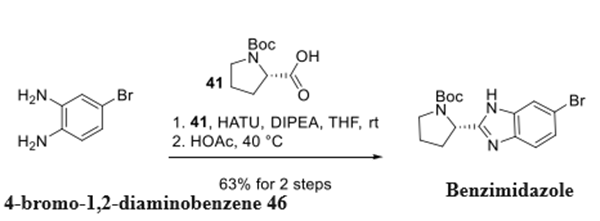
Synthesis of Ravidasvir Benzimidazole Fragment
Benzimidazole intermediate was synthesized in two steps from 4-bromo-1,2-diaminobenzene (46) via amide formation with N-Boc-L-proline (41) followed by cyclization in warm glacial acetic acid.