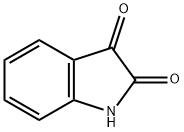
Synthesis of Isatin
Isatin was first of all reported by Erdman and Laurent in 1841 as a new product from the oxidation of indigo by nitric and chromic acids. Its utility was recognized as an intermediate for the construction of a variety of heterocycles to generate molecular diversity as well as in pharmaceutical chemistry due to diverse biological properties. In nature, isatin is found in plants of the genus Isatis in Calanthe discolor LINDL and in Couroupita guianensis Aubl. Its presence has also been ascertained in the parotid gland of Bufo frogs, and in humans as a metabolic derivative of adrenaline. Isatin has also been found as a component from coal tar.

Physical Properties
Parent isatin is a bright orange-colored stable crystalline solid, basic in nature, with an mp of 204°C. The IR spectrum of parent isatin shows two strong bands at 1740 and 21620 cm–1 due to carbonyl stretching vibrations. One broad band at 3190 has been assigned for N-H stretching frequency and is modified depending on the N-substitution. The 1H NMR spectrum in DMSO D6 showed peaks at 6.91 (d, J = 7.83 Hz), 7.07 (t, J = 7.55 Hz), 7.50 (d, J = 7.59 Hz), and 7.59 (t, J = 7.71 Hz) ppm for H7, H5, H4, and H6 protons, respectively. The electron donating N-substituent does not affect the chemical shifts of ring protons, while electron-withdrawing substituents shift the NMR signals to downfield.188 The 13C NMR spectrum in DMSO-d6 showed eight signals at δ 184.6, 159.5, 150.9, 138.5, 124.8, 122.9, 117.9, and 112.4 ppm corresponding to eight carbon atoms.
Chemical Reactivity
There are three different sites in isatins for chemical reactions, namely N-substitution, aromatic ring substitution at C5, and carbonyl reaction at the C3 site. In the case of electron-withdrawing substituents in the benzene ring or at the ring nitrogen, reactions occur at the C2 site.
Synthesis
Sandmeyer Reaction
The frequently used and oldest method for the synthesis of isatin was developed by Sandmeyer.176 It is a three-component reaction of aniline, chloral hydrate, and hydroxylamine hydrochloride in aqueous sodium sulfate to yield 2-(hydroxyimino)-N-phenylacetamide, which on acid treatment with sulfuric acid furnished isatin.

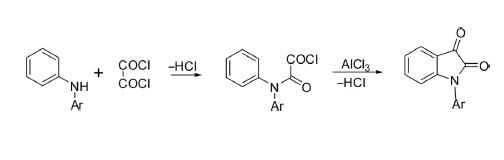
Stolle Reaction
The methodology reported by Stolle for the synthesis of isatin is an alternative to the Sandmeyer reaction. This approach is the reaction of N-substituted aniline with oxalyl chloride to form chlorooxalylanilide as an intermediate, which is cyclized in the presence of Lewis acid to produce isatin. This protocol is useful for the synthesis of N-alkyl/ arylisatin.
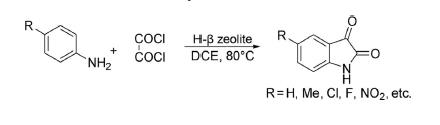
An alternative to the Stolle reaction, one-pot synthesis of isatin has been developed by Raj et al.178 from the reaction of aniline with oxalyl chloride in the presence of reusable catalyst H-β zeolite using dichloroethane as a solvent at 80°C under heterogeneous conditions in 48%–79% yields.
You may like
Related articles And Qustion
Lastest Price from Isatin manufacturers

US $0.00/kg2025-08-22
- CAS:
- 91-56-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $10.00/KG2025-04-21
- CAS:
- 91-56-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons