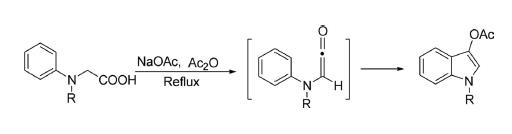
Synthesis of Indoxyl
Indoxyl exists in two possible tautomeric equilibria with keto and enol forms in which latter is the minor component. The pKa of indoxyl is 10.46 and clearly indicates its more acidic nature than 2-oxindole (pKa ~18). The anion generated from indoxyl is also in equilibrium with the oxygen anion and carbon anion as per tautomeric forms and accordingly offers both types of products on reaction with electrophiles.

Physical Properties
Indoxyl is a yellow crystalline solid with an mp of 85°C and is an important intermediate for the synthesis of indigo. As already mentioned, the ketonic tautomeric form of indoxyl is predominant and C2 protons are acidic in nature and preferentially give condensation and alkylation reactions readily. Chemical Reactivity Alkylation of indoxyl in aqueous NaOH and solid NaOH gives O-alkylation and C-alkylation products due to its enol and keto tautomeric forms.
Synthesis
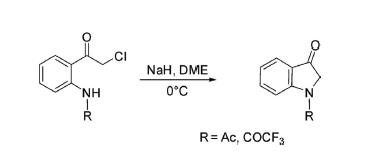
1 2-Chloro-1(2-alkylamino)phenyl)ethan-1-one derived from arylamine underwent cyclization through intramolecular displacement reaction in the presence of NaH in dimethoxyethane (DME) at 0°C delivered N-substituted indolin-3-one.
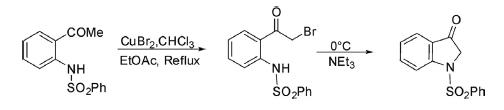
2 N-Phenylsulfonyl-1,2-dihydro-3H-indol-3-one has been synthesized173 from 2-bromoacetyl-N-(phenylsulfonyl) aniline by intramolecular base-catalyzed cyclization at 0°C.
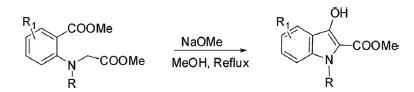
Methyl 2-(N-alkyl-N-carbomethoxymethyl)aminobenzoate intramolecularly cyclized in the presence of NaOMe in methanol at reflux temperature to 2-carbomethoxy-3-hydroxy-N-substituted indole.
N-Methyl-N-phenylglycine HCl and sodium acetate in a mixture of acetic anhydride and DMF was heated at
reflux for 3 h. Evaporation of solvent and treatment of residue with water and extraction with DCM gave the desired
compound.