Synthesis of 2-Oxindole
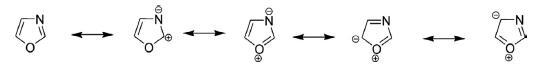
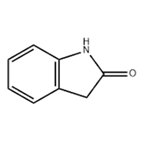
2-Oxindole is a bicyclic nitrogen heterocycle with a carbonyl function at position 2 of the fused pyrrole ring, having two possible tautomeric forms: lactam and lactim. The lactim tautomeric form is highly unstable and undetectable even at low temperature, while the lactam form is quite stable. The C3 protons of 2-oxindole are acidic in nature and readily deprotonated resulting in anions and stabilized by resonating contributors. It readily reacts with electrophiles like alkyl halides and gives aldol condensation products reacting with carbonyl compounds. The substructure of oxindole is present in numerous naturally occurring pharmacologically active oxindole alkaloids such as the hexacyclic cage-like compound gelsemine and highly cytotoxic (−)-spyrotryprostatin B.
Related chemical reactions
2-Oxindole undergoes Claisen condensation with diethyl carbonate and gives 3-ethoxycarbonyl-2-oxindole.

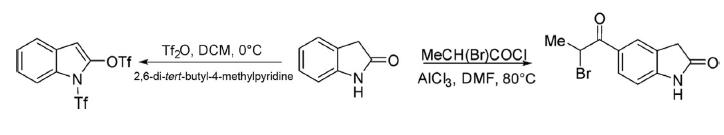
Reaction of 2-bromopropanoyl chloride with 2-oxindole in the presence of anhydrous AlCl3 in DMF at 80°C underwent a Friedel-Crafts acylation reaction in the aromatic ring providing 5-(2-bromopropanoyl)-indolin-2-one. 2-Oxindole on reaction with triflic anhydride in the presence of 2,6-di-tert-butyl-4-methylpyridine in DCM at 0°C gave 1-trifluoromethylsulfonyl-2-trifluoromethylsulfonyloxy-indole.
Synthesis
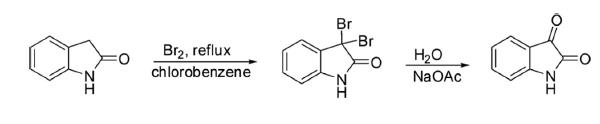
2-Oxindole is oxidized to 1H-indol-2,3-dione through bromination in chlorobenzene at reflux temperature with the formation of 3,3-dibromoindolin-2-one as an intermediate followed by hydrolysis with aqueous sodium acetate.
Lastest Price from Oxindole manufacturers
US $0.00-0.00/KG2025-04-04
- CAS:
- 59-48-3
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton

US $9.00/KG2024-10-11
- CAS:
- 59-48-3
- Min. Order:
- 1KG
- Purity:
- 99.9
- Supply Ability:
- 1 ton