Synthesis of Ensartinib Dihydrochloride
Synthesis of Ensartinib Dihydrochloride
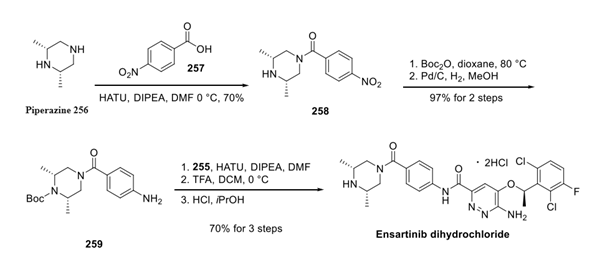
Ensartinib Dihydrochloride is synthesised by a three-step reaction using piperazine as a raw material and the addition of the intermediate compounds Ensartinib Amino Pyridazine and Ensartinib Carboxylic Acid. The specific synthesis steps are as follows:
Step 1: Preparation of Ensartinib Amino Pyridazine
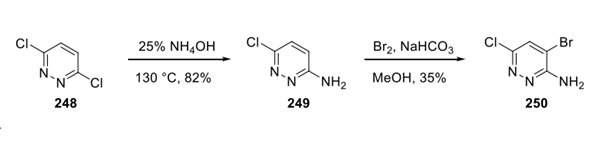
First, SNAr of dicholoropyridazine 248 with ammonium hydroxide furnished amino-pyridazine 249 in 82% yield. The treatment of 249 with bromine under basic conditions provided the dihalogenated pyridazine building block 250 in 35% yield.
Step 2: Preparation of Ensartinib Carboxylic Acid
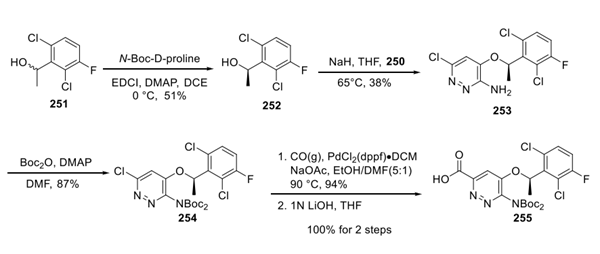
For the second building block, a mixture of benzylic alcohols (251) was resolved using N-Boc-D-proline and standard esterification conditions to deliver alcohol 252 in 51% yield. No enantiopurity measurements were reported. Exposure of this system to sodium hydride in THF led to an unusual substitution reaction with bromide 250. Presumably, the regiochemical outcome of this reaction can be rationalized by the energetics imparted by the vicinal amino-bromide situated about the pyridazine ring within 250. Relief of the 1,2-strain in the transition state likely drives preferential substitution at the carbon bearing the bromide, but the yields of the chlorosubstituted substrate (or other byproducts from this reaction) were not reported by Xcovery. After ether 253 was recovered in 38% yield, the amine underwent bis-Boc protection followed by a metal-catalyzed carbonylative insertion in ethanol to arrive at an ester that was then saponified to provide acid 255.
Step 3: Preparation of Ensartinib Dihydrochloride
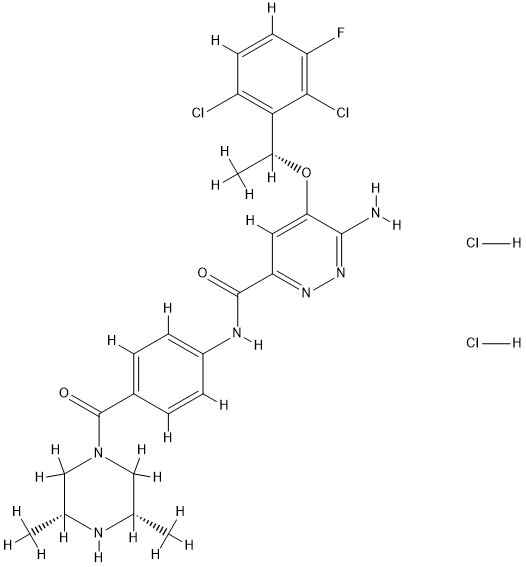
For the third building block, piperazine 256 was first reacted with 257 under coupling conditions to form amide 258, which then underwent Boc protection followed by nitro reduction to arrive at aniline 259. Finally, coupling with acid 255 followed by the treatment with isopropanolic HCl furnished ensartinib as a dihydrochloride salt.