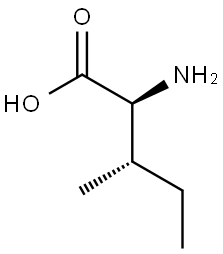
Synthesis of DL-Isoleucine
Isoleucine (symbol Ile or I)is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms).
It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes in other organisms such as bacteria.
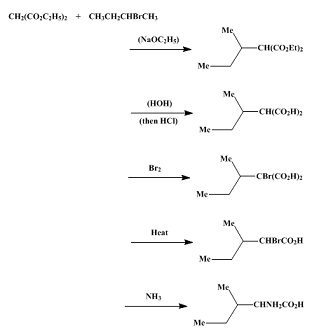
Synthesis of DL-Isoleucine
A. Diethyl sec.-butylmalonate.
To 700 ml. of absolute ethanol in a 2-l. three-necked round-bottomed flask equipped with a long, wide-bore reflux condenser is added 35 g. (1.52 gram atoms) of sodium cut in pieces of suitable size. When all the sodium has reacted, the flask is placed on a steam cone and fitted with a mercury-sealed stirrer, a dropping funnel, and a reflux condenser bearing a calcium chloride tube. The flask is heated, and 250 g. (1.56 moles) of diethyl malonate is added in a steady stream with stirring.
After the ester addition, 210 g. (1.53 moles) of sec-butyl bromide is added at such a rate that the heat of reaction causes refluxing. The mixture is then stirred and refluxed for 48 hours. At the end of this time, the reflux condenser is exchanged for a downward condenser and the ethanol is removed by distillation . The residue is treated with 200 ml. of water, shaken, and allowed to stand until the ester layer separates. The ester layer is separated from the aqueous layer and distilled from a 500-ml. two-necked flask fitted with a well-wrapped 18-in. Vigreux column. The fraction boiling at 110–120°/18–20 mm. is diethyl sec-butylmalonate, and it amounts to 274–278 g. (83–84%).
B. α-Bromo-β-methylvaleric acid.
The cooling is hastened by the addition of 50 g. of ice, and, when the temperature reaches 15°, technical hydrochloric acid is added at such a rate that the temperature does not rise above 20°. After the addition of about 250 ml. of acid, the monopotassium salt separates, necessitating stirring by hand until solution again occurs. When the solution is acid to Congo red it is transferred to a separatory funnel .
The sec-butylmalonic acid is extracted with three 200-ml. portions of ether, and the combined extracts are dried over calcium chloride overnight. The ether solution is then decanted into a 2-l. three-necked flask fitted with a mercury-sealed stirrer, reflux condenser, and dropping funnel. Five milliliters of bromine is added at one time, and the solution is stirred until decolorized. Then 50 ml. more bromine is added dropwise at such a rate that the ether refluxes gently. When all the bromine has been added, 200 ml. of water is added through the dropping funnel dropwise so as to produce no foaming or violent reaction.
The ether layer containing the bromomalonic acid is separated from the aqueous layer, and the ether is removed by distillation from a steam cone. The residual liquid is decarboxylated by refluxing for 5 hours in a 500-ml. round-bottomed flask on an oil bath heated to 130°. The bromo acid is then separated from the small amount of water and distilled. The material distilling at 125–140°/18–20 mm. is α-bromo-β-methylvaleric acid. The yield is 150.5 g. (66.7%).
C. dl-Isoleucine.
One hundred and fifty grams (0.77 mole) of α-bromo-β-methylvaleric acid is added to 645 ml. of technical ammonium hydroxide (sp. gr. 0.90) in a 1.5-l. round-bottomed flask. A stopper is wired in, and the flask is allowed to stand at room temperature for a week. The stopper is removed and the mixture heated on a steam cone overnight to remove ammonia. The aqueous solution is concentrated under reduced pressure until bumping becomes violent (about 300 ml.). The mixture is then cooled to 15° and the crystals are collected on a filter. The crystals are washed with 40 ml. of ethanol and dried. The filtrate is again concentrated to about 150 ml., and a second crop of crystals is obtained. This second crop is washed with 25 ml. of water and then with 25 ml. of 95% ethanol. The yield of crude product is 65 g.
The isoleucine is recrystallized by dissolving it in 850 ml. of water heated to 95° on a steam cone. The solution is decolorized by treatment with a gram of Norit for 30 minutes and is then filtered hot. To the hot solution is added 425 ml. of 95% ethanol. and the flask is placed in an ice chest overnight. The yield of pure product is 38 g. An additional crop of 12 g. may be obtained by concentrating the mother liquors from the recrystallization to about 100 ml. and adding an equal volume of ethanol. This second crop is washed with 10 ml. of cold water and 10 ml. of cold ethanol. The total yield is 50 g. (49%). The product decomposes at 278–280° in a sealed evacuated capillary.
You may like
See also
Lastest Price from DL-Isoleucine manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 443-79-8
- Min. Order:
- 1KG
- Purity:
- 98%min;food grade
- Supply Ability:
- 500kgs

US $10.00/KG2025-04-21
- CAS:
- 443-79-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt