Synthesis of Cedazuridine
Synthesis of Cedazuridine
Cedazuridine is synthesised using gemcitabine precursor as raw material by chemical reaction. The specific synthesis steps are as follows:
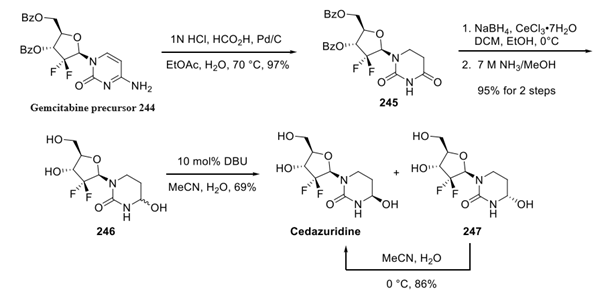
Beginning with the readily accessible, protected gemcitabine precursor 244. Conversion of the 6-amino-pyridone to the corresponding dihydrouridine was accomplished through the use of acidmediated transfer hydrogenation to generate 245 in 97% yield. Reduction under Luche conditions and subsequent exposure to methanolic ammonia reduced the amide carbonyl and removed the two benzoyl ester protecting groups to provide dihydrouridine 246 as a mixture of two diastereomers. Treatment of 246 with catalytic DBU in aqueous acetonitrile resulted in a diastereomeric mixture of cedazuridine and its epimeric aminol 247 in a 9:1 ratio, respectively. Resubjection of the undesired diastereomer 247 to a 5:1 mixture of chilled acetonitrile/water provided cedazuridine in 86% yield.
Introduction of Cedazuridine
Cedazuridine is a cytidine deaminase inhibitor that was discovered by Astex Pharmaceuticals and developed by Taiho Oncology. Cedazuridine was approved by the USFDA for use in combination with decitabine for the treatment of myelodysplastic syndromes, including chronic myelomonocytic leukemia. Cedazuridine-mediated inhibition of cytidine deaminase increases the half-life of decitabine, therefore increasing the efficacy of the combination therapy as compared to the monotherapy alone.