Synthesis of Capmatinib Dihydrocholride
Synthesis of Capmatinib Dihydrocholride
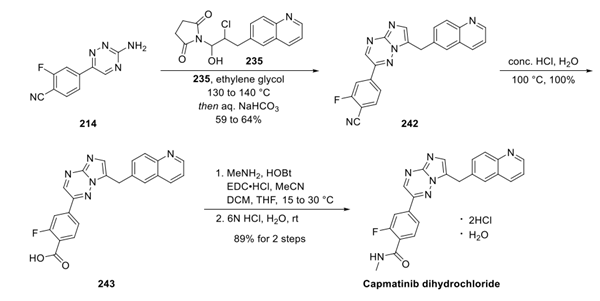
Capmatinib Dihydrocholride is synthesised from a quinoline fragment and a bis-aryl fragment of capmatinib by a late cyclisation reaction. The specific synthesis steps are as follows:
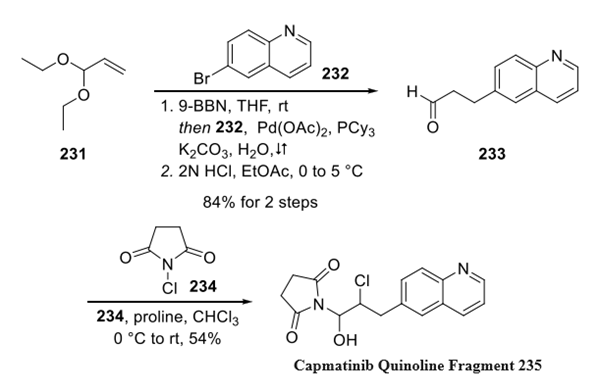
Step 1: Preparation of Capmatinib Quinoline Fragment
For the preparation of quinoline fragment 235, acrolein diethyl acetal 231 was treated with 9- BBN, and the in situ generated alkyl borane was subjected to Suzuki cross-coupling with 6-bromoquinoline 232. The Suzuki coupling product was converted to aldehyde 233 under aqueous acid conditions. Hemiaminal 235 was synthesized by the treatment of aldehyde 233 with N-chlorosuccinimide (NCS, 234) in the presence of a catalytic amount of proline.
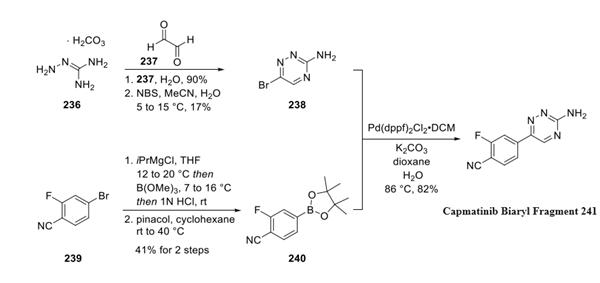
Step 2: Preparation of Capmatinib Biaryl Fragment
The synthesis of biaryl fragment 241 began with a condensation reaction between aminoguanidine bicarbonate 236 and glyoxal 237 to generate 1,2,4- triazin-3-amine, which was subsequently brominated to furnish 6-bromo-1,2,4-triazin-3-amine 238 in 17% yield. The authors did not comment on the suboptimal yield obtained in the bromination step. Concurrently, boronate coupling partner 240 was prepared from commercially available 4-bromo-2-fluoro-benzonitrile 239 via halogen-metal exchange and electrophilic trapping with trimethyl borate. A Suzuki coupling between bromo-triazine 238 and aryl boronate 240 delivered biaryl fragment 241 in 82% yield.
Step 3: Preparation of Capmatinib Dihydrocholride
First, fragments 235 and 241 were united via an annulation reaction in warm ethylene glycol to generate imidazo-triazine 242, which was hydrolyzed under aqueous acidic conditionsto provide benzoic acid 243. Finally, amidation and treatment with 6 N HCl delivered capmatinib as the monohydrate dihydrochloride salt.