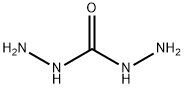
Synthesis of carbohydrazide
Hydrazine does not contribute solids to the system, so boiler blowdown, or the mechanical removal of solids from the after-boiler section as sludge, is reduced. It also promotes the formation of the protective magnetite film on the boiler tubes and drum, and converts red iron dust (Hematite) to magnetite. It is because of these passivation effects that an excess of scavenger to oxygen is required when changing a boiler system form a non-passivating scavenger to one which passivates. It is worth mentioning that hydrazine is not without limitations. It is not considered “volatile”, so it does not leave the boiler with the steam to scavenge oxygen and passivate metal throughout the system. In boilers operating above 400 °F (205 °C), it can degrade to ammonia and volatilize with steam, and, in the presence of oxygen, attack metals containing copper. Finally, the important point is the inclusion of hydrazine on the OSHA and NIOSH lists as a suspect carcinogen. Carbohydrazide which is a volatile oxygen scavenger contributes no solids to the system, reacts readily with oxygen at low temperatures and pressures, and passivates the metal of the boiler system. Carbohydrazide can break down to hydrazine above temperatures of 350 °F (180 °C) to scavenge oxygen, but this conversion is not necessary for oxygen scavenging activity because it reacts directly with oxygen. Therefore, due to the better oxygenation properties, higher roughness and lower toxicity of this compound than hydrazine; it is now considered as one of the suitable alternatives for replacing hydrazine in boilers. In addition, due to the solid and non toxicity of transport and storage, carbohydrazide is safer and easier than hydrazine. NALCO has now introduced this substance in water-soluble formulation under the trademark "ELIMIN-OX" to the chemical market . The cobalt complex of carbohydrate has medicinal properties in and uses.
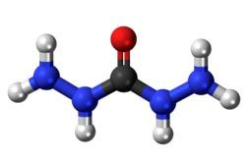
The employing of carbohydrazide instead of hydrazine has many advantages such as: I) evaluating the suitability and efficiency of carbohydrazide as an alternative oxygen scavenger to hydrazine in the high-pressure boiler. II) determining the consequences of degradation byproducts on boiler system. III) evaluating the ability of the alternative oxygen scavenger in forming and maintaining an oxide film in the boiler. IV) evaluating whether the alternative oxygen scavenger is generating any negative effects on the efficiency of the boiler or not. One of the most commonly methods for the production of carbohydrazide is the reaction of dialkyl carbonate (dimethyl, ethyl, normal propyl, isopropyl or normal butyl carbonate) and hydrazine hydrate in two steps . The basis of this method is a nucleophilic attack of hydrazine on electron deficient carbon. During this reaction, the alkoxy group is displaced by ‒NHNH2. In this study, the production of the mentioned compound as a substitute for hydrazine was performed in the process of decontamination of boiler water and, in fact, solving the environmental problem and the risks of using hydrazine were its immediate results.
Experimental
Materials and methods
All chemicals applied in this work were prepared from Fluka (Buchs, Switzerland) and were employed without additional purification. In this research, hydrazine hydrate (100%) hydrazine (64%) and dimethyl carbonate as a source of dialkyl carbonate are employed for preparation of
carbohydrazide.
Preparation of oxzole derivatives
To a stirred mixture of carbohydrazide (2 mmol) and ketone (2 mmol) after 45 min was added activated acetylenic compounds (2 mmol) slowly. After After completion of the reaction (5h; TLC control (n-hexane/AcOEt, 6:1), water (15 mL) was poured in the mixture and solide filtered and washed with cold ether to afforded pure oxazole.
Preparation of methyl hydrazinicarboxylate
To prevent contact with the contents of the container with air, before performing the reaction,the nitrogen gas is blown into the pot and also during the process; the reaction is carried out under at mospheric nitrogen gas. In order to react, a three glass spheroid balloon with a volume of 1Liter used. To a stirred mixture of hydrazine hydrate (0.5 mol) and dimethyl carbonate (0.5 mol) at 50-75 °C nitrogen gas is blown:
CH3OCOOCH3 + N2H4.H2O → NH2NHCOOCH3 + CH3OH + H2O
Regarding the stoichiometry of reaction, hydrazine is as a limiting agent, and therefore, the absence of hydrazine in the vapor phase represents the end point of the reaction. The hydrazine in the vapor phase is evaluated by UV spectroscopy. For this purpose, the steam phase is sampled at the same time intervals and is supplemented with p-dimethyl amino-benzaldehyde (I). If hydrazine is present in the sample, it is converted to azine (III), and is seen in bright yellow.
(CH3)2NC6H4CH=O + N2H4 → (CH3)2NC6H4CH=NNH2 (I) (II)
(CH3)2NC6H4CH=NNH2 + (CH3)2NC6H4CH=O → CH3)2NC6H4CH=NN=CHC6H4N (CH3)2 (III)
To detect the end of the reaction by the UV device, the absorbance is measured at 485 nm. Upon
completion of the reaction and in order to remove water and methanol, distillation is used under vacuum at ambient temperature. The distillation is carried out at the appropriate vacuum pressure and finally, methylhydrazinocarboxylate with a purity of 94.5% and a melting point of 73 °C is prepared.
Preparation of carbohydrazide from methyl hydrazinocarboxylate Preparation of carbohydrazide from methylhydrazinocarboxylate is performed according to the following reaction:
NH2NHCOOCH3 + N2H4.H2O → NH2NHCONHNH2 + CH3OH + H2O
In this stage, hydrazine hydrate (1.1 mol) was added to methyl hydrazinicarboxylate at 70 °C.Steam phase sampling shows that the hydrazine concentration which is almost constant indicates completion of the reaction. The reaction solution is then cooled to 0 °C in which the crystalline carbohydrazide is crystallized and is filtered by Büchner funnel. For purification, the carbohydrazide was washed with ethanol and dried in vacuum at 80 °C for 1h. The process efficiency based on the percentage of dimethyl carbonate conversion to carbohydrate is 75%.
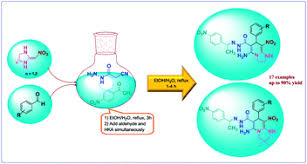
Results and discussion
The reaction of carbohydrazide 1, activated acetylenic compounds 2 and ketone 3 produce oxazole derivatives 4 under solvent-free conditions at room temperature in good yield.
The infrared spectrum of carbohydrazide was displayed in stretching vibration band of carbonyl group in 1639.4 cm-1, bending vibration of N‒H in 1539 cm-1 and a bending vibration of N‒H in the second type amide in 3357.8 cm-1 and 3303.8 cm-1. Accordingly, it confirmed the spectrum provided for carbohydrazide in the IR reference.
CHN analysis results of this compound are presented in Table 1. According to the chemical formula of carbohydrazide, this composition should be contained 13.33% Carbon, 6.71% Hydrogen and 62.19% Nitrogen.
Conclusion
In summary, oxazol derivatives was prepared via the reaction of carbohydrazide, electron deficient acetylenic compounds and ketone ingood yield. Also, because of harmful effects of hydrazine on the human body and the environment, the use of this substance has been reduced and extensive measures are being taken to replace it with non-toxic substances. The production process used in this research is carried out under relatively comfortable operating conditions (Temperature and pressure) and, in comparison to other methods of production, its raw materials are toxic and more hazardous. The results of the experiments on the prepared samples indicate the production of carbohydrazide with high purity.
Related articles And Qustion
See also
Lastest Price from Carbohydrazide manufacturers

US $0.00/kg2025-11-13
- CAS:
- 497-18-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500mt/year

US $10.00-6.00/kg2025-10-31
- CAS:
- 497-18-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 600tons