Synthesis of Benzoxazoles
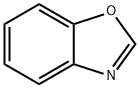
Benzoxazole is an important class of heterocycle having a bicyclic structure in which an aryl ring is fused with the “d” site of an oxazole moiety. The parent benzoxazole scaffold has a planar geometry and is found to be a constituent of several naturally occurring molecules. This chemical entity often exhibits affinity and selectivity toward various biological targets and shows a number of noncovalent interactions since both oxygen and nitrogen atoms can act as hydrogen acceptors. In addition, the presence of a planar benzene ring in benzoxazole offers π–π stacking, π-cation, and hydrophobic interactions with biomacromolecules. Therefore this scaffold is often described as the building blocks for the synthesis of various pharmacologically relevant molecules.
Physical Properties
It is a yellow-orange solid with an mp of 28–30°C, a bp of 182–185°C, and a density of 1.1754 gm/cm3. It is slightly soluble in water, with a pKb of 13.2 and heat of combustion –of 3445 kJ/mol.
Synthesis
By considering the biological significance of benzoxazoles, numerous procedures for the synthesis of these molecules have been developed in the past. Among these, the most general synthetic strategies are discussed next.
1. From ortho-Aminophenols
A number of one-pot synthetic methods are reported by employing ortho-aminophenols as one of the key starting materials. These procedures also include eco-friendly protocols for making benzoxazoles. Condensation of o-aminophenols with aldehydes by using samarium triflate as a reusable acid catalyst under aqueous conditions affords the corresponding benzoxazoles. Similarly, these analogs have been prepared by the reaction of o-aminophenols with a wide range of substrates such as benzoic acids, benzoyl chlorides, aldehydes, β-diketones, aryl halides, isocyanides, alcohols, and N-cyano-N-phenyl-p-toluenesulfonamide-halogenated nitriles under diverse conditions as presented in the following scheme.
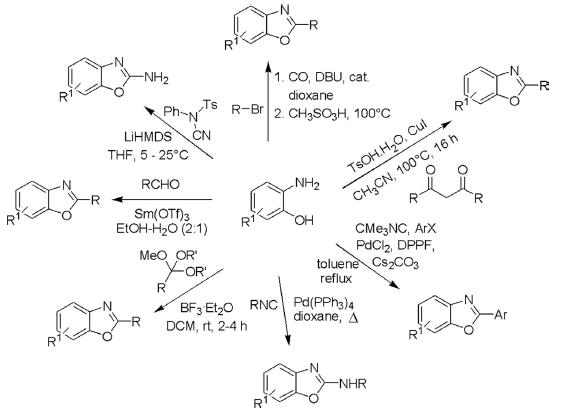
2. From ortho-Hydroxyacetophenones
These substrates react with trimethylsilylazides in the presence of ZrCl4 or TfOH in dichloromethane and form imine diazonium salt as an intermediate, which on aryl migration followed by intramolecular cyclization afforded corresponding benzoxazole derivatives.

3. From ortho-Bromoarylamides
2-Bromoarylamides undergo intramolecular cyclization in the presence of a base such as K2CO3 or Cs2CO3 and either 5 mol% CuO nanoparticles in DMSO at 110°C or CuI and 1,10-phenanthroline as catalyst under conventional heating conditions to form a variety of benzoxazoles.

You may like
Lastest Price from Benzoxazole manufacturers

US $15.00-10.00/KG2021-07-13
- CAS:
- 273-53-0
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton

US $15.00-10.00/KG2021-07-10
- CAS:
- 273-53-0
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton