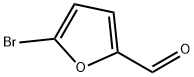
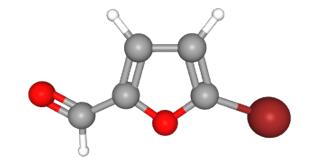
Synthesis of 5-Bromo-2-furfural under Solvent-Free Conditions
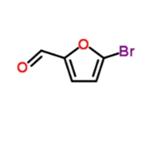
5-Bromo-2-furfural (CAS: 1899-24-7) with the molecule formula of C5H3BrO2 is key intermediate in the preparation of important biologically active compounds , pharmaceutical and agrochemical[1]. In previous reports 5-bromo2-furfural was generally synthesized using 2-furfural as starting material, which was subjected to bromination with equivalents of bromine either in a solvent or solely in the presence of aluminum chloride. Molecular bromide, the simplest brominating agent, which has high atom utilization and widely application in large-scale industrial production though, suffers from several drawbacks of hazardous and difficult to handle, especially from poor regioselectivity. These reactions in many cases result in a mixture of mono and dibrominated products. In spite of the present methodologies, there is still a desire to explore a mild, efficient and environmentally benign route for the highly selective preparation of 5-bromo-2-furfural [2].
Reaction in solvent-free and ionic liquids medium is attracting increasing interest for good selectivity, enhanced reaction rate, manipulative simplicity and friendly environment as an alternative to classical molecular solvents in synthetic organic chemistry. Herein, an efficient synthetic process of high selectivity for the monobromination of 2-furfural using 1-butyl-3-methylimidazolium tribromide ([bmim]Br3) as brominating agent as compared with other brominating agents.
Melting points were determined on digital melting point apparatus and were uncorrected. TLC analyses were carried out on silica gel 60 GF254 precoated aluminum sheets using UV light for detection. HPLC (Shimadzu LC-10 Avp Plus using 30 volume of distilled water to 70 volume of methanol as eluent.) was utilized to determine product compositions and substrate conversions using n-dodecane as internal standard. IR spectra were recorded in cm-1 (KBr) (Hitachi IR meter 260-10). 1H NMR spectra were recorded on a Bruker 400 MHz spectrometer using the indicated solvents.
Preparation of 1-butyl-3-methylimidazolium bromide ([bmim]Br):
A three-necked 50 mL round bottom flask was charged with N-methylimidazole (0.10 mol) followed by dropwise addition of 1-bromobutane (0.11 mol) at 70 ºC. After the addition was completed, the resulting mixture was stirred at 140 ºC for 1.5 h. The combined organic layer was washed three times by ethyl acetate and vacuum-dried overnight at 70 ºC.
Preparation of 1-butyl-3-methylimidazolium tribromide ([bmim]Br3)
Bromine (0.10 mol) was added in dropwise to stirred 1-butyl-3-methylimidazolium bromide (0.10 mol), which obtained by the above experiment. After the addition was completed, the resulting mixture was stirred for 2 h at room temperature and washed by ethyl acetate (30 mL × 3). The residue was vacuum-dried overnight at 70 ºC.
A typical preparation of 5-bromo-2-furfural:
A three-necked 50 mL round bottom flask was charged with [bmim]Br3 (20 mmol) followed by dropwise addition of freshly distilled 2-furfural (20 mmol) over a period of 0.5 h and the reaction mixture was stirred for at 40 ºC 2.5 h under nitrogen atmosphere. The mixture was extracted with petroleum ether (30 mL × 3), the organic layer was washed with water, dried (sodium sulfate) and concentrated. The crude product was purified by distillation and recrystallization with 10 % ethyl acetate-ether solution. 5-Bromo-2-furfural of yellowish crystal in 88 % yield was obtained. Mp. 82-85 ºC, 1H NMR (CDCl3), δ: 9.55 (s, 1H), 7.25 (s, 1H), 6.57 (s, 1H).
In conclusion, the bromination of 2-furfural with [Bmim]Br3, could occur rapidly under solvent-free conditions at 40 ºC and completed within 3 h with high yield. 1-Butyl-3-methylimidazolium tribromide ([Bmim]Br3), is an important alternative to the use of traditional brominating agents for its simplicity, high selectivity, high reactivity and environmentally more benign [2].
Reference
[1]https://pubchem.ncbi.nlm.nih.gov/compound/5-Bromo-2-furaldehyde#section=Related-Compounds-with-Annotation
[2] Wu, X., X. Peng, X. Dong and Z. Dai (2012). "Synthesis of 5-Bromo-2-furfural under Solvent-Free Conditions Using 1-Butyl-3-Methylimidazolium Tribromide as Brominating Agent." Asian Journal of Chemistry 24: 927-928.
Lastest Price from 5-Bromo-2-furaldehyde manufacturers
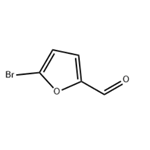
US $0.00-0.00/kg2025-04-04
- CAS:
- 1899-24-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton
US $0.00/kg2024-03-28
- CAS:
- 1899-24-7
- Min. Order:
- 25kg
- Purity:
- 98%
- Supply Ability:
- Inquiry