Synthesis of 1,2,3-Oxadiazole
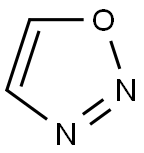
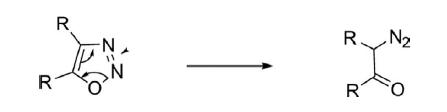
1,2,3-Oxadiazole is a five-membered, unsaturated, unstable, nonbenzenoid, heteroaromatic, comprised of two carbon atoms, one oxygen atom, and two pyridine-type nitrogen atoms linked in continuity (O-N-N). Depending on the position of the nitrogen atoms, the four possible regioisomeric structures are generated for oxadiazoles. The cyclic structure for 1,2,3-oxadiazoles is unstable and readily isomerizes to α-diazoketone, an open chain structure.
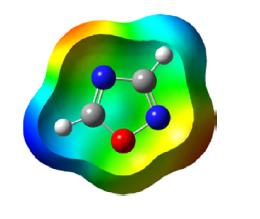
Fusion of the aromatic ring to the 1,2,3-oxadiazole ring does not provide stability to the molecule. However, the 1,2,3-oxadiazole ring system present in sydnone (mesoionic) and sydnonimine is quite stable.
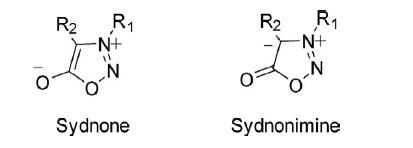
The ring atoms of sydnone contribute seven 2pz electrons to the resonating structures and one 2pz electron on the exocyclic atom. Once one of the 2pz electrons of the ring is paired with a single electron on the exocyclic oxygen atom, a sextet of electrons is formed, which is characteristic of the aromatic character of the ring. Thus the ring is positively charged and balanced by a negatively charged exocyclic atom.
Physical Properties
3-Phenylsydnone is a crystalline compound prone to hydrolysis with ring cleavage, especially in basic medium. The carbonyl stretching frequency in the IR spectra of sydnones is found in the range of 1720–1790 cm–1 depending on the substituent present. The UV spectra of alkylsydnones showed a single maxima at 290 nm. The chemical shifts in the 1H and 13C NMR spectra of 3-phenylsydnone confirmed the dipolar structure of sydnones. The C4H proton resonated at 6.78 ppm in D2O, while 3-phenyl protons appeared downfield at 7.70 ppm due to positively charged nitrogen.
13C NMR of 3-methylsydnone, δ (ppm): C4, 97.7; C5, 170.7, CH3, 40.4.
Synthesis
1. 3-Phenylsydnone has been synthesized by nitrosation of amino acid followed by ring closure in the presence of a dehydrating agent to yield the final product. In this reaction, trifluoroacetic anhydride, phosphorous oxychloride, and thionyl chloride, etc. are used as dehydrating agents.
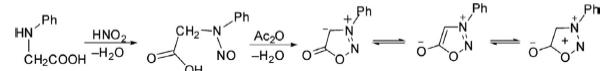
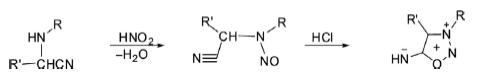
2. Sydnonimines are synthesized by the nitrosation of α-aminonitriles followed by acid-induced ring closure.