Chemical Reactivity of 1,2,4-Oxadiazole
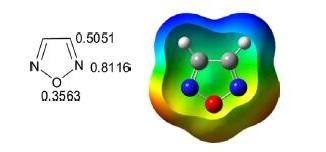
1,2,4-Oxadiazole is a five-membered, planar, conjugated, nonbenzonoid, aromatic heterocyclic system comprised of one oxygen atom and two nitrogen atoms at positions 2 and 4 of the oxadiazole ring. The oxadiazole ring has two pyridine-like nitrogen atoms. Positions 3 and 5 are vulnerable for nucleophilic substitution to form 3,5-disubstituted 1,2,4-oxadiazoles. The topography of the 1,2,4-oxadiazole ring reveals that it has a more heterodiene character compared to its aromatic nature. The substituted 1,2,4-oxadiazole ring system was first reported in 1880 but the parent 1,2,4-oxadiazole was not reported until 1962 because of instability of the molecule.
There are diverse pharmacological activities associated with 1,2,4-oxadiazoles, e.g., bioisosteres of esters and amide, potent muscarinic agonists, antirhinovirals, antitussives, anthelmintics, antihistamine H3 receptor antagonists, anesthetics, analgesics, antiinflammatory agents, liquid crystals, and agrochemicals as plant protecting agents.
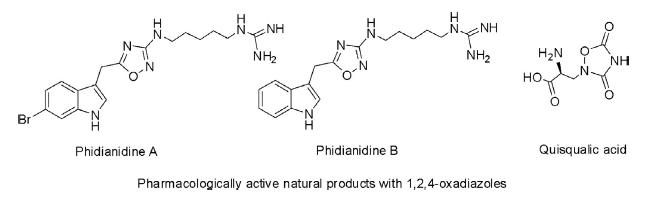
Pharmacologically Active Natural Products
The 1,2,4-oxadiazole ring system is present in various natural products of pharmacological importance. Phidianidine A and phidianidine B are isolated from the aeolid opisthobranch Phidiana militaris and are selective inhibitors of the dopamine transporter. The other natural product, quisqualic acid, isolated from the seeds of Quisqualis indica and Quisqualis fructus is a strong agonist for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors.
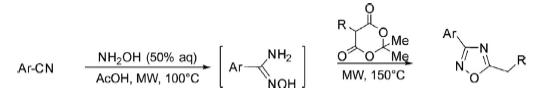
Synthesis From Nitriles
One-pot synthesis of 3,5-disubstituted-1,2,4-oxadiazoles has been reported900 from the reaction of nitriles, hydroxylamine, and Meldrum’s acids under microwave irradiation and solvent-free conditions in good to excellent yields.
Physical Properties
The parent 1H-1,2,4-oxadiazole is highly unstable and its synthesis has not been reported so far. The 3,5-disubstituted 1,2,4-oxadiazoles are stable, while monosubstituted oxadiazoles are less stable and prone to acid- and base-induced hydrolysis. The 13C NMR spectra of 3,5-diaryl-1,2,4-oxadiazoles in CDCl3 showed chemical shift for C3 at 168.8–169.0 ppm and C5 at 174.7–175.8 ppm.
Chemical Reactivity
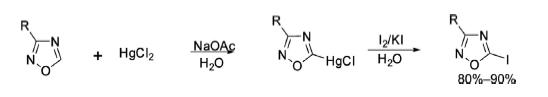
The C3- and C5-positions of 1,2,4-oxadiazoles are almost inert for electrophilic substitution because of the topography of heteroatoms in the cyclic ring system. As a result, halogenation, nitration, acylation, and alkylation do not occur in the oxadiazole ring. However, electrophilic mercuration takes place in 5-unsubstituted 1,2,4-oxadiazoles, which acts as a good precursor for iodination with iodine/KI to make 5-iodo-3-substituted 1,2,4-oxadiazoles.