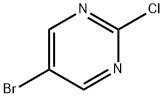
Synthesis Methods of 5-Bromo-2-chloropyrimidine
5-Bromo-2-chloropyrimidine is a halogenated pyrimidine analogue containing chloro and bromo substituents at positions 2 and 5 of the benzene ring structure. 5-Bromo-2-chloropyrimidine is applied as a pharmaceutical intermediate, in the synthesis of pharmaceutical goods such as inhibitors. It is also employed in the cross-coupling reactions of indium organometallics.

Synthesis from 5-bromo-2-hydroxypyrimidine
5-Bromo-2-chloropyrimidine is an intermediate in the synthesis of compounds. In the prior art, 5-bromo-2-hydroxypyrimidine is generally obtained by reacting 2-hydroxypyridine with bromine, and then prepared by reacting 5-bromo-2-hydroxypyrimidine with phosphorus oxychloride. Bromine has high toxicity, troublesome post-processing, and low product yield. A novel synthesis of [2-14C]2,5-dichloropyrimidine (By Tran, Scott B. et al From Journal of Labelled Compounds and Radiopharmaceuticals, 54(13), 813-815; 2011) discloses a synthesis using phosphorus oxychloride, But post-processing is more troublesome. The preparation method of 5-bromo-2-chloropyrimidine of the present invention avoids the use of phosphorus oxychloride, because phosphorus oxychloride is highly toxic, the post-reaction treatment is cumbersome, and there are many three wastes. When the preparation method of 5-bromo-2-chloropyrimidine of the present invention uses 5-bromo-2-hydroxypyrimidine to synthesize, the chlorination reagent is hydrochloric acid, and cetyltrimethylammonium chloride is used as a catalyst, and the by-product is less, the post-processing is simple, the yield is high, and the purity can reach 99%. The preparation method of 5-bromo-2-chloropyrimidine of the present invention uses 2-hydroxypyridine to synthesize 5-bromo-2-hydroxypyrimidine, selects tribromopyridine as the brominating reagent, avoids the use of bromine which is more toxic, and after-treatment Simple, high yield and high purity.[1]
The preparation method of 5-bromo-2-chloropyrimidine of the present embodiment comprises the following steps: 1)Preparation of 5-bromo-2-hydroxypyrimidine: take a 500mL three-necked flask, add 9.6g of 2-hydroxypyrimidine and 100mL of toluene, start stirring, replace argon three times, add 48.0g of tribromopyridine, and place the reaction flask on the Stir in an oil bath at 80°C for 8 hours, drop to 25°C, add 100 mL of water, and separate the layers. The aqueous layer is extracted twice with ethyl acetate, the organic phases are combined, and the organic phase is washed twice with 10% sodium thiosulfate solution, saturated salt Washed with water once, dried over anhydrous sodium sulfate, concentrated to dryness, then added 30 mL of ethyl acetate, started stirring, added 300 mL of n-heptane, stirred at 25 °C for 8 h, filtered, and dried in vacuo to obtain 14 g of 5-bromo-2-hydroxyl Pyrimidine, yield 80%. 2)Preparation of 5-bromo-2-chloropyrimidine: take a dry 500mL three-necked bottle, add 14g of 5-bromo-2-hydroxypyrimidine and 280mL of DMF, start stirring, add 5.12g of cetyltrimethylammonium chloride and 20 mL of 6 mol.L-1 hydrochloric acid, then the three-necked flask was placed in an oil bath at 40 °C, stirred for 12 h, lowered to 0 °C, adjusted to pH 6 with saturated sodium bicarbonate solution, extracted three times with ethyl acetate, The organic phases were combined, washed once with saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 14.1 g of 5-bromo-2-chloropyrimidine with a yield of 91% and a purity of 99.76%.
Preparation of 5-bromo-2-chloropyrimidine from 2-chloropyrimidine:
The technical problem to be solved by the present invention is to provide a novel method for preparing 2-aminopyrimidine-5-boronic acid pinacol ester, comprising the following steps: In the first step, under the catalysis of BF3-Et2O, 2-chloropyrimidine and NBS are refluxed in a solvent of acetonitrile or ethylene glycol dimethyl ether to obtain 5-bromo-2-chloropyrimidine; In the second step, 2.5M n-BuLi is added to n-BuMgBr at a temperature of -20 to -10 ° C to prepare n-Bu3MgLi, and then the 5-bromo-2-chloropyrimidine obtained in the first step is maintained at this temperature. Dissolved in tetrahydrofuran, dropped into the above solution, kept for 1 hour, dropwise added pinacol methoxyborate or pinacol isopropoxide, kept for 1 hour, the solution was raised to room temperature, stirred overnight, and saturated chlorine was added. Quenching with ammonium, extracting with ethyl acetate, and evaporating to give 2-chloropyrimidine-5-boronic acid pinacol ester; In the third step, the 5-bromo-2-chloropyrimidine acid pinacol ester obtained in the second step is added to ammonia water or ammonia methanol in an autoclave, and reacted at 80 to 100 ° C for 6 hours, and the solution is spin-dried and then added with tetrahydrofuran or acetic acid. The ethyl ester was dissolved, filtered, and the solvent was evaporated again, and then recrystallized from methanol to give 2-aminopyrimidine-5-boronic acid pinacol.[2]
Reductive deamination of aromatic amines
General procedure: A solution of amine 2 (5 mmol) in THF (3 mL) was added dropwise over 20 min to a solution of t-BuONO (7.5 mmol) and DMSO (0.5 mmol) in THF (7 mL) at 30 °C. The mixture was stirred at 30 °C until the starting materials 2 were consumed (monitored by TLC). The solvent was evaporated, and the yields of the low boiling point products 5-bromo-2-chloropyrimidine were determined by GC; the high boiling point products were isolated by column chromatography on silica gel (hexane/ethyl acetate).[3]
References
[1]ZHENGZHOU CAT EYE AGRICULTURAL SCIENCE AND TECH - CN114644594, 2022, A
[2]CANGZHOU PURUI DONGFANG TECHNOLOGY - CN104788482, 2016, B
[3]Fang, Lu; Qi, Liang; Ye, Longfei; Pan, Zhentao; Luo, Wenjun; Ling, Fei; Zhong, Weihui[Journal of Chemical Research, 2018, vol. 42, # 11, p. 579 - 583]
See also
Lastest Price from 5-Bromo-2-chloropyrimidine manufacturers
US $0.00-0.00/kg2025-08-26
- CAS:
- 32779-36-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $100.00-2500.00/KG2025-05-19
- CAS:
- 32779-36-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2MT per month