Sustainable Pathways for L-Lactide Production
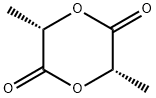
L-Lactide (3,6-dimethyl-1,4-dioxane-2,5-dione) is a cyclic ester used as an intermediate product in the production of poly(lactic acid) (PLA), the desired monomer for high-molar mass PLA synthesis. This dimer has a high cost in the market, due to its expensive and time-consuming process for its synthesis. Factors such as racemization, low selectivity, and high energy consumption are associated with its production procedure.

Cyclic l-lactide synthesis from lignocellulose biomass
Cyclic chiral lactide is the monomer chemical for polymerization of high molecular weight polylactic acid (PLA). The synthesis of cyclic l-lactide starts from poly-condensation of l-lactic acid to a low molecular weight prepolymer and then depolymerized to cyclic l-lactide. Lignocellulose biomass is the most promising carbohydrate feedstock for lactic acid production, but the synthesis of cyclic l-lactide from l-lactic acid produced from lignocellulose has so far not been successful. The major barriers are the impurities of residual sugars and inhibitors in the crude cellulosic l-lactic acid product. Here we show a successful cyclic l-lactide synthesis from cellulosic l-lactic acid by lignocellulose biorefining with complete inhibitor removal and coordinated sugars assimilation. The removal of inhibitors from lignocellulose pretreatment was accomplished by biodetoxification using a unique fungus Amorphotheca resinae ZN1. The nonglucose sugars were completely and simultaneously assimilated at the same rate with glucose by the engineered l-lactic acid bacterium Pediococcus acidilactici. The l-lactic acid production from wheat straw was comparable to that from corn starch with high optical pure (99.6%), high l-lactic acid titer (129.4 g/L), minor residual total sugars (~2.2 g/L), and inhibitors free. The cyclic l-lactide was successfully synthesized from the regularly purified l-lactic acid and verified by detailed characterizations. This study paves the technical foundation of carbon-neutral production of biodegradable PLA from lignocellulose biomass.[1]
The impurity existence of residual inhibitors and nonglucose sugars strongly restricts the consequent poly-condensation of cellulosic l-lactic acid as well as the depolymerization of the prepolymer to cyclic l-lactide. This study shows a successful high-titer l-lactic acid production from wheat straw feedstock by a dry biorefinery processing and a subsequent cyclic l-lactide synthesis using the cellulosic l-lactic acid. Dry acid pretreatment preserved the nonglucose sugars along with all inhibitors in the lignocellulose feedstock, and the inhibitors were removed by biodetoxification using a unique kerosene fungus Amorphotheca resinae ZN1 to CO2 and water without loss of fermentable sugars. An engineered l-lactic acid bacterium Pediococcus acidilactici ZY271 completely and coordinately converted the total lignocellulose derived glucose and nonglucose sugars (xylose, arabinose, mannose, and galactose) into high-titer and high-charity cellulosic l-lactic acid. The cellulosic l-lactic acid obtained after simultaneous saccharification and co-fermentation (SSCF) of pretreated and biodetoxified wheat straw was nearly completely free of residual sugars and inhibitors. The cyclic l-lactide was successfully synthesized using cellulosic l-lactic acid purified by regular methods for the first time and characterized as polymerization grade l-lactide after regular purification steps. This study paves the way for production of the carbon-neutral biodegradable polymer PLA from abundant lignocellulose biomass.
The cyclic l-lactide synthesis using the obtained cellulosic l-lactic acid was conducted with only regular purification steps applied, and without resorting to techniques such as molecular distillation. The purified l-lactic acid was prepolymerized and depolymerized into cyclic l-lactide using the regular stannous octoate catalyst and the synthesized cyclic l-lactide was characterized in details. The main purpose of this study was to evaluate the feasibility of this material synthesis from cellulosic l-lactic acid, therefore the experimental setup for l this material synthesis (a 250 ml three-port glass flask) was not suitable for the quantitative determination of l-lactide product. Considerable this material was accumulated in the entry port section and the cooling tube of the setup, due to the insufficient insulation for recovering the synthesized l-lactide by vaporization at 240–260°C. Only small portion of this material (~10%–20% of the total) was recovered in the receiving flask and it is practically not possible to collect the condensed this material solids from the cooling glassware for l-lactide yield calculation. Both cellulosic and commercial l-lactic acid used showed the same phenomenon. More accurate quantitation of this material synthesis will be conducted after the setup and catalyst are improved.
Synthesis of L-Lactide from Lactic Acid and Production of PLA Pellets
Poly(L-lactide) is also widely used for the production of biodegradable packaging. The stereoisomers of lactide are shown. The copolymerization of different stereoisomers is an effective way to control the properties and degradation profile of PLA. Therefore, the form determines what kind of product will be obtained. To synthesize pure L-lactide, it is required to use L-lactic acid and sustain the reaction conditions that minimize the production of meso-lactide. Namely, it is the necessary to maintain the temperature and pressure regime and use catalysts that allow the necessary stereospecificity to be achieved. Tin octoate was chosen as the optimal catalyst for this reaction based on the literature data because it has stereoselective properties that would allow to purer L-lactide to be obtained while minimizing the formation of D-form and meso-lactide.[2]
L-lactide was synthesized by depolymerization of PLA oligomers with the introduction of tin octoate at different concentrations into the system as a catalyst. It was found that the optimal amount of catalyst is about 0.50 wt.%. The yield after four-fold recrystallization was 47%, and the presence of lactic acid was not detected. It was also shown, by the DSC method, that after four recrystallization iterations, the melting point of lactide stopped changing and amounted to 97.2 °C. The thermograms also lack peaks not associated with the melting of L-lactide. The data obtained by GPC confirmed that the purity degree of the obtained L-lactide is sufficient for the synthesis of high-molecular-weight PLA, where Mw > 200 kDa. The weight average molecular weight of the synthesized lactide was 228 kDa, and the polydispersity index was 1.94. The resulting molecular weight of the polymer is sufficient to synthesize copolymers of various molecular weights and structures based on purified lactide.
Properties of L-Lactide
In a work carried out for PLGA synthesis by ROP, the characterization of the commercial L-lactide monomer (Purasorb® L) used in the development of the research was carried out. The data obtained showed that L-lactide degrades completely at a temperature of 233 °C observed by thermogravimetric analysis, presenting a single stage of mass loss in the thermal profile. The infrared absorption analysis (FTIR) of the L-lactide shows strong absorption bands characteristics of L-lactide at approximately 935 cm−1 (ester group C-COO stretching), 1056 cm−1 (COO ring deformation), 1093 cm−1 (C-C stretching), 1240 cm−1 (symmetric COC lactone ring stretching), 1266 cm−1 (CH2 and CH wagging), and 1754 cm−1 (asymmetric CH3 deformation). The X-ray diffractogram presented in the same work shows the crystalline microstructure of L-lactide with intense peaks at approximately 2Ɵ equal to 8, 13, and 14° Complementing the lactide characterization topic, its nuclear magnetic resonance analysis (1H NMR) shows two signals in the L-lactide spectrum, one corresponds to a doublet at δ 1.6 ppm, and the other presents a quadruplet at δ 5.0 ppm, that are respective to the methine (CH) and methyl (CH3) groups of the L-lactide molecule The other lactide isomers present chemical shifts close to the values presented for the L-lactide, with a doublet signal at δ 1.4–1.8 ppm for D- and meso-lactides. Some efforts to differentiate and quantify the lactide isomers via chromatographic techniques have been published.[3]
References
[1]He N, Jia J, Qiu Z, Fang C, Lidén G, Liu X, Bao J. Cyclic l-lactide synthesis from lignocellulose biomass by biorefining with complete inhibitor removal and highly simultaneous sugars assimilation. Biotechnol Bioeng. 2022 Jul;119(7):1903-1915.
[2]Aliev G, Toms R, Melnikov P, Gervald A, Glushchenko L, Sedush N, Chvalun S. Synthesis of L-Lactide from Lactic Acid and Production of PLA Pellets: Full-Cycle Laboratory-Scale Technology. Polymers (Basel). 2024 Feb 25;16(5):624.
[3]Cunha BLC, Bahú JO, Xavier LF, Crivellin S, de Souza SDA, Lodi L, Jardini AL, Filho RM, Schiavon MIRB, Concha VOC, Severino P, Souto EB. Lactide: Production Routes, Properties, and Applications. Bioengineering (Basel). 2022 Apr 7;9(4):164.
Lastest Price from L-Lactide manufacturers
US $0.00-0.00/KG2025-12-01
- CAS:
- 4511-42-6
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $30.00/kg2025-04-21
- CAS:
- 4511-42-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100 tons