Synthesis, Detection method and Application of 2'-Deoxyuridine
General description
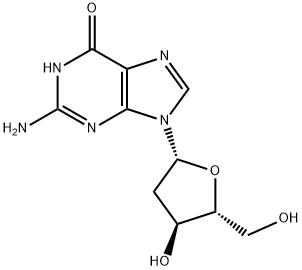
2'-Deoxyuridine is a natural deoxynucleoside, which can be directly used to prepare combined deoxynucleoside drugs or used as chemical reagents for biochemical research. At the same time, it can be used as an intermediate to synthesize some antiviral nucleoside drugs and molecular markers, such as 8-bromo-2-deoxyuridine and 8-hydroxy-2-deoxyuridine from 2'-Deoxyuridine.
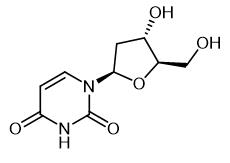
Fig. 1 The structure of 2'-Deoxyuridine.
Physicochemical property
2'-Deoxyuridine is a white or off-white powdered solid with a melting point of 167 to 169 °C. Its boiling point is roughly estimated to be 370.01°C. It is slightly soluble in water, DMSO, and slightly soluble in methanol when heated.
Synthetic routes
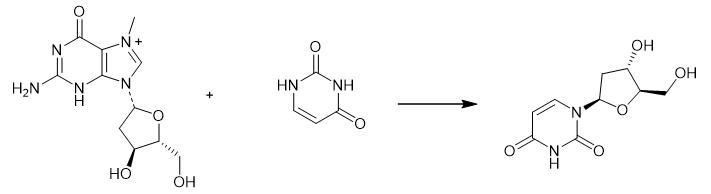
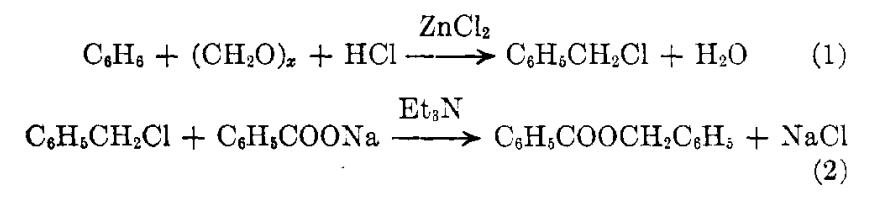
Fig. 2 The synthetic method 1 of 2'-Deoxyuridine.
Dissolve uracil (196 mg, 1.75 mmol) in 100 mL of 50 mM Tris-HCl buffer (pH 7.5) under heating and cool the solution to room temperature. Add potassium dihydrophosphate (119 mg, 0.875 mmol) and the reactant (1.074 g, 2.62 mmol) to the solution. Dilute the resulting mixture with 50 mM Tris-HCl buffer (pH 7.5) to a volume of 350 mL. Add solutions containing enzymes E. coli PNP [10 μL of 32mg/mL solution of E. coli PNP (2.95 U)] and E. coli TP [10 μL of 17.2 mg/mL solution of E. coli TP diluted 10-fold (1.2 U)] to the mixture using a dispenser at room temperature. Stir the resulting mixture for 5 min and allow to stand overnight at room temperature without stirring. Filter the reaction mixture through a Phenomenex membrane (0.2 m, 47mm) and concentrate under vacuum to a volume of ca. 50 mL. Filter the suspension from the precipitate of 7-methylguanine through a Phenomenex membrane (0.2 m, 47 mm) and concentrate under vacuum to near dryness. Dissolve the residue in ethanol (50 mL) and add silica gel (20 mL) to the solution. Evaporate the resulting mixture to dryness, co-evaporate with ethanol (2 X 50 mL) and apply the dry residue on a chromatographic column (4 X 10 cm) packed with silica gel (150 mL) for purification. Wash the column with a mixture of dichloromethane and ethanol (95 :5 v/v, 200 mL). Elute the product with dichloromethane :ethanol (90 :10 v/v) and dichloromethane :ethanol (85 :15 v/v). Collect the fractions containing the product and evaporate under vacuum to dryness. Purify the resulting residue on a chromatographic column (2 X 10 cm) packed with silica gel (50 mL). Wash the column with adichloromethane:ethanol mixture (95 :5 v/v, 200 mL). Elute the product with dichloromethane :ethanol (90 :10 v/v). Collect the fractions containing the product. Evaporate the product under vacuum, co-evaporate 5times with dichloromethane and dry on a vacuum pump at room temperature for 1 hour [1].
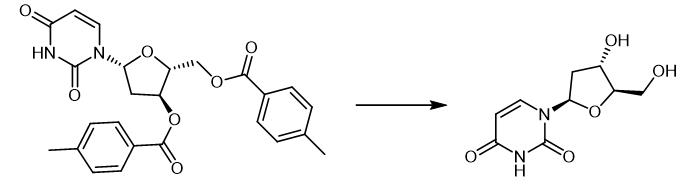
Fig. 3 The synthetic method 2 of 2'-Deoxyuridine.
The precursor 3,5-(toluoyl)-2-deoxy-(N1,N3-15N)-uridine (1) was synthesized according to Schiesser et al.4 In a round bottom flask 1 (76 mg, 0.16 mmol, 1.0 eq.) was dissolved in dry MeOH (2.1 mL) and K2CO3 (49 mg, 0.35 mmol, 2.2 eq.) was added. The suspension was stirred at 40 °C for 6 h. The solvent was removed by rotary evaporation. The residue was then suspended in H2O (5 mL) and extracted with DCM (5 mL). The aqueous layer was then concentrated to dryness, redissolved in H2O and subjected to RP-HPLC (0% to 20% MeCN in water in 45 min, 5 mL/min). [15N2]-dU as a white solid (31 mg, 0.13 mmol, 84%). 1H-NMR (400 MHz, [D6]-DMSO, ppm): δ = 11.27 (d, J=89.3 Hz, 1H, 15N-H), 7.84 (dd, J=8.1, 2.0 Hz, 1H, 15N-CH), 6.15 (t, J=6.7 Hz, 1H, O-CH-N), 5.62 (ddd, J=7.9, 4.5, 2.5 Hz, 1H, CH-C=O), 5.23 (s, 1H, OH), 5.00 (s, 1H, OH), 4.27-4.20 (m, 1H, O-CH2-CH-CH-O), 3.79-3.76 (m, 1H, O-CH2-CH-O), 3.59 - 3.51 (m, 2H, O-CH2-CH-O), 2.13-2.02 (m, 2H, N-CH-CH2). 13C-NMR (100 MHz, [D6]-DMSO, ppm): δ = 163.1 (d, J=9.2 Hz, C=O-C), 150.4 (dd, J=18.1, 17.6 Hz, 15N-C=O-15N), 140.5 (d, J=12.3 Hz, 15N-CH=CH), 101.7 (d, J=6.4 Hz, N-CH=CH), 87.4 (CH-CH2-O), 84.1 (d, J=12.1 Hz, N-CH-O), 70.4 (d, J=0.9 Hz, CH-CH-O), 61.3 (CH2-O), 40.4 (CH2-CH-N). 15N-NMR (40 MHz, [D6]-DMSO, ppm): δ = -233.1, -222.4. HRMS (ESI-): calc. for C9H1115N2O5 -[M-H]-: 229.0614, found: 229.0616. IR (ATR): υ (cm-1) = 3266 (w), 3127 (w), 3001 (w), 2936 (w), 2795 (w), 1660 (s), 1450 (m), 1388 (m), 1367 (m), 1250 (s), 1179 (m), 1098 (s), 1046 (s), 998 (s), 958 (s), 860 (s), 760 (s), 626 (s). Melting range: > 250 °C decomposition. UV (H2O, 161 μM): λmax = 262 nm [2].
Detection method
A novel method employing high-performance liquid chromatograph-mass spectrometry (LC-MS) has been developed and validated for the quantitation of plasma 2 '-deoxyuridine (UdR). It involves a plasma clean-up step with strong anion-exchange solid-phase extraction (SAX-SPE) followed by HPLC separation and atmospheric pressure chemical ionization mass spectrometry detection (APCI-MS) in a selected-ion monitoring (SIM) mode. The ionization conditions were optimised in negative ion mode to give the best intensity of the dominant formate adduct [M + HCOO](-) at m/z 273. Retention times were 7.5 and 12.5 min for 2 '-deoxyuridine and 5-iodo-2 '-deoxyuridine, an iodinated analogue internal standard (IS), respectively. Peak area ratios of 2 '-deoxyuridine to IS were used for regression analysis of the calibration curve. The latter was linear from 5 to 400 nmol/l using 1 ml sample volume of plasma. The average recovery was 81.5% and 78.6% for 2 '-deoxyuridine and 5-iodo-deoxyuridine, respectively. The method provides sufficient sensitivity, precision, accuracy and selectivity for routine analysis of human plasma 2 '-deoxyuridine concentration with the lowest limit of quantitation (LLOQ) of 5 nmol/l. Clinical studies in cancer patients treated with the new fluoropyrimidine analogue capecitabine (N-4-pentoxycarbonyl-5 '-5-fluorocytidine) have shown that plasma 2 '-deoxyuridine was significantly elevated after 1 week of treatment, consistent with inhibition of thymidylate synthase (TS). These findings suggest that the mechanism of antiproliferative toxicity of capecitabine is at least partly due to TS inhibitory activity of its active metabolite 5-fluoro-2 '-deoxyuridine monophosphate (FdUMP). Monitoring of plasma UdR concentrations have the potential to help clinicians to guide scheduling of capecitabine or other TS inhibitors in clinical trials. Marked differences of plasma 2 '-deoxyuridine between human and rodents have also been confirmed. In conclusion, the LC-MS method developed is simple, highly selective and sensitive and permits pharmacodynamic studies of TS inhibitors in several species [3].
Application
Preparation of E-5-(2-bromovinyl)-2'-deoxyuridine
The potent anti-herpes agent, E-5-(2-bromovinyl)-2'-deoxyuridine was conveniently synthesized via four steps of hydroxylmethylation, selective oxidation, Knoevenagel condensation and Hunsdiecker reaction, from commercially available 2'-deoxyuridine with unprotected
Related articles And Qustion
See also
Lastest Price from 2'-Deoxyguanosine manufacturers
US $10.00-99.00/Kg2025-10-10
- CAS:
- 961-07-9
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00/kg2025-08-27
- CAS:
- 961-07-9
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons